Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells
- PMID: 18802069
- PMCID: VSports最新版本 - PMC2810501
- DOI: 10.4049/jimmunol.181.7.4666
Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells
VSports - Abstract
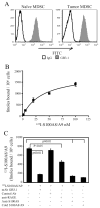
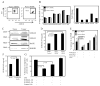
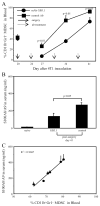
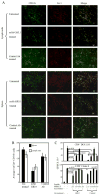
Chronic inflammation is a complex process that promotes carcinogenesis and tumor progression; however, the mechanisms by which specific inflammatory mediators contribute to tumor growth remain unclear. We and others recently demonstrated that the inflammatory mediators IL-1beta, IL-6, and PGE(2) induce accumulation of myeloid-derived suppressor cells (MDSC) in tumor-bearing individuals. MDSC impair tumor immunity and thereby facilitate carcinogenesis and tumor progression by inhibiting T and NK cell activation, and by polarizing immunity toward a tumor-promoting type 2 phenotype. We now show that this population of immature myeloid cells induced by a given tumor share a common phenotype regardless of their in vivo location (bone marrow, spleen, blood, or tumor site), and that Gr1(high)CD11b(high)F4/80(-)CD80(+)IL4Ralpha(+/-)Arginase(+) MDSC are induced by the proinflammatory proteins S100A8/A9. S100A8/A9 proteins bind to carboxylated N-glycans expressed on the receptor for advanced glycation end-products and other cell surface glycoprotein receptors on MDSC, signal through the NF-kappaB pathway, and promote MDSC migration. MDSC also synthesize and secrete S100A8/A9 proteins that accumulate in the serum of tumor-bearing mice, and in vivo blocking of S100A8/A9 binding to MDSC using an anti-carboxylated glycan Ab reduces MDSC levels in blood and secondary lymphoid organs in mice with metastatic disease. Therefore, the S100 family of inflammatory mediators serves as an autocrine feedback loop that sustains accumulation of MDSC. Since S100A8/A9 activation of MDSC is through the NF-kappaB signaling pathway, drugs that target this pathway may reduce MDSC levels and be useful therapeutic agents in conjunction with active immunotherapy in cancer patients VSports手机版. .
Conflict of interest statement
The authors have no financial conflict of interest.
Figures


References
-
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. - PubMed
-
- Ruegg C. Leukocytes, inflammation, and angiogenesis in cancer: fatal attractions. J Leukocyte Biol. 2006;80:682–684. - PubMed
-
- Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-κB in cancer cells converts inflammation- induced tumor growth mediated by TNFα to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305. - PubMed
-
- Karin M, Greten FR. NF-κB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. - VSports手机版 - PubMed
V体育官网 - Publication types
- "VSports在线直播" Actions
"V体育2025版" MeSH terms
- "VSports最新版本" Actions
- "V体育官网入口" Actions
- "VSports app下载" Actions
- VSports - Actions
- VSports手机版 - Actions
- V体育官网 - Actions
- "VSports手机版" Actions
- V体育2025版 - Actions
- VSports注册入口 - Actions
- VSports手机版 - Actions
- "V体育ios版" Actions
- V体育安卓版 - Actions
- Actions (V体育安卓版)
- "VSports app下载" Actions
- Actions (V体育官网入口)
Substances
- "V体育2025版" Actions
Grants and funding
LinkOut - more resources
"VSports在线直播" Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

