Amylase-binding protein B of Streptococcus gordonii is an extracellular dipeptidyl-peptidase
- PMID: 18678669
- PMCID: PMC2546850 (V体育官网)
- DOI: 10.1128/IAI.00186-08 (VSports在线直播)
V体育平台登录 - Amylase-binding protein B of Streptococcus gordonii is an extracellular dipeptidyl-peptidase
Abstract
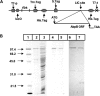
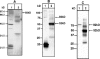
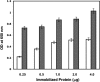
The oral commensal bacterium Streptococcus gordonii interacts with salivary amylase via two amylase-binding proteins, AbpA and AbpB. Based on sequence analysis, the 20-kDa AbpA protein is unique to S. gordonii, whereas the 82-kDa AbpB protein appears to share sequence homology with other bacterial dipeptidases. The aim of this study was to verify the peptidase activity of AbpB and further explore its potential functions. The abpB gene was cloned, and histidine-tagged AbpB (His-AbpB) was expressed in Escherichia coli and purified VSports手机版. Its amylase-binding activity was verified in an amylase ligand binding assay, and its cross-reactivity was verified with an anti-AbpB antibody. Both recombinant His-AbpB and partially purified native AbpB displayed dipeptidase activity and degraded human type VI collagen and fibrinogen, but not salivary amylase. Salivary amylase precipitates not only AbpA and AbpB but also glucosyltransferase G (Gtf-G) from S. gordonii supernatants. Since Streptococcus mutans also releases Gtf enzymes that could also be involved in multispecies plaque interactions, the effect of S. gordonii AbpB on S. mutans Gtf-B activity was also tested. Salivary amylase and/or His-AbpB caused a 1. 4- to 2-fold increase of S. mutans Gtf-B sucrase activity and a 3- to 6-fold increase in transferase activity. An enzyme-linked immunosorbent assay verified the interaction of His-AbpB and amylase with Gtf-B. In summary, AbpB demonstrates proteolytic activity and interacts with and modulates Gtf activity. These activities may help explain the crucial role AbpB appears to play in S. gordonii oral colonization. .
Figures


References
-
- Banas, J. A., and M. M. Vickerman. 2003. Glucan-binding proteins of the oral streptococci. Crit. Rev. Oral Biol. Med. 1489-99. - V体育官网 - PubMed
-
- Burnette, W. N. 1981. “Western blotting”. Electrophoretic transfer of proteins from sodium dodecyl sulphate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 112195-203. - PubMed
-
- Choih, S., Q. T. Smith, and C. F. Schachtele. 1979. Modification of human parotid saliva by oral Streptococcus sanguis. J. Dent. Res. 58516-523. - PubMed
Publication types
- Actions (VSports)
"VSports注册入口" MeSH terms
- Actions (V体育官网入口)
- VSports app下载 - Actions
- Actions (VSports最新版本)
- Actions (VSports最新版本)
- "V体育2025版" Actions
- VSports手机版 - Actions
- Actions (V体育平台登录)
- "V体育2025版" Actions
- V体育官网入口 - Actions
- VSports app下载 - Actions
- V体育官网入口 - Actions
- V体育官网入口 - Actions
- Actions (V体育安卓版)
"VSports app下载" Substances
- Actions (V体育ios版)
- "VSports app下载" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

