"V体育平台登录" Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms
- PMID: 18656388
- PMCID: VSports app下载 - PMC2847796
- DOI: "V体育ios版" 10.1016/j.immuni.2008.05.016
"VSports最新版本" Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms
Abstract
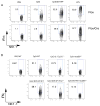
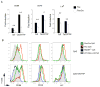
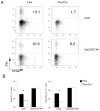
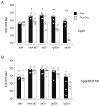
Toll-like receptors (TLRs) play prominent roles in initiating immune responses to infection, but their roles in particular cell types in vivo are not established. Here we report the generation of mice selectively lacking the crucial TLR-signaling adaptor MyD88 in dendritic cells (DCs) VSports手机版. In these mice, the early production of inflammatory cytokines, especially IL-12, was substantially reduced after TLR stimulation. Whereas the innate interferon-gamma response of natural killer cells and of natural killer T cells and the Th1 polarization of antigen-specific CD4(+) T cells were severely compromised after treatment with a soluble TLR9 ligand, they were largely intact after administration of an aggregated TLR9 ligand. These results demonstrate that the physical form of a TLR ligand affects which cells can respond to it and that DCs and other innate immune cells can respond via TLRs and collaborate in promoting Th1 adaptive immune responses to an aggregated stimulus. .
"VSports注册入口" Conflict of interest statement
The authors declare they have no competing financial interests.
Figures


"V体育平台登录" Comment in
-
2008: A MyD88 O, DC.Immunity. 2008 Aug 15;29(2):173-5. doi: 10.1016/j.immuni.2008.07.006. Immunity. 2008. PMID: 18701079
VSports注册入口 - References
-
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. - PubMed
-
- Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–557. - PubMed
-
- Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J Immunol Methods. 1990;132:191–195. - PubMed
-
- Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1–16. - PubMed
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
Publication types
- Actions (V体育官网入口)
"V体育平台登录" MeSH terms
- "VSports注册入口" Actions
- "VSports" Actions
- Actions (VSports手机版)
- "V体育2025版" Actions
- Actions (V体育平台登录)
- "VSports app下载" Actions
- Actions (VSports)
- VSports手机版 - Actions
- VSports app下载 - Actions
- Actions (V体育ios版)
- VSports最新版本 - Actions
- "VSports注册入口" Actions
- "V体育2025版" Actions
- "VSports app下载" Actions
- VSports注册入口 - Actions
- Actions (V体育官网)
- V体育官网 - Actions
- "V体育平台登录" Actions
Substances
- "V体育官网" Actions
- VSports - Actions
- Actions (VSports)
- Actions (V体育官网)
Grants and funding
"V体育ios版" LinkOut - more resources
"VSports手机版" Full Text Sources
"V体育安卓版" Other Literature Sources
"V体育2025版" Molecular Biology Databases
Research Materials

