Translational regulation of glutathione peroxidase 4 expression through guanine-rich sequence-binding factor 1 is essential for embryonic brain development
- PMID: 18593884
- PMCID: PMC2492670
- DOI: 10.1101/gad.466308
Translational regulation of glutathione peroxidase 4 expression through guanine-rich sequence-binding factor 1 is essential for embryonic brain development
Abstract
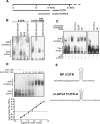
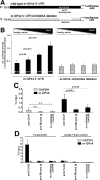
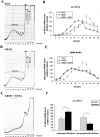
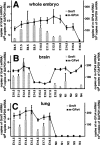
Phospholipid hydroperoxide glutathione peroxidase (GPx4) is a moonlighting selenoprotein, which has been implicated in basic cell functions such as anti-oxidative defense, apoptosis, and gene expression regulation. GPx4-null mice die in utero at midgestation, and developmental retardation of the brain appears to play a major role. We investigated post-transcriptional mechanisms of GPx4 expression regulation and found that the guanine-rich sequence-binding factor 1 (Grsf1) up-regulates GPx4 expression. Grsf1 binds to a defined target sequence in the 5'-untranslated region (UTR) of the mitochondrial GPx4 (m-GPx4) mRNA, up-regulates UTR-dependent reporter gene expression, recruits m-GPx4 mRNA to translationally active polysome fractions, and coimmunoprecipitates with GPx4 mRNA. During embryonic brain development, Grsf1 and m-GPx4 are coexpressed, and functional knockdown (siRNA) of Grsf1 prevents embryonic GPx4 expression. When compared with mock controls, Grsf1 knockdown embryos showed significant signs of developmental retardations that are paralleled by apoptotic alterations (TUNEL staining) and massive lipid peroxidation (isoprostane formation). Overexpression of m-GPx4 prevented the apoptotic alterations in Grsf1-deficient embryos and rescued them from developmental retardation. These data indicate that Grsf1 up-regulates translation of GPx4 mRNA and implicate the two proteins in embryonic brain development VSports手机版. .
Figures
References
-
- Bernstein D.S., Buter N., Stumpf C., Wickens M. Analyzing mRNA–protein complexes using a yeast three-hybrid system. Methods. 2002;26:123–141. - PubMed
-
- Borchert A., Savaskan N.E., Kuhn H. Regulation of expression of the phospholipid hydroperoxide/sperm nucleus glutathione peroxidase gene. Tissue-specific expression pattern and identification of functional cis- and trans-regulatory elements. J. Biol. Chem. 2003;278:2571–2580. - PubMed
-
- Borchert A., Wang C.C., Ufer C., Schiebel H., Savaskan N.E., Kuhn H. The role of phospholipid hydroperoxide glutathione peroxidase isoforms in murine embryogenesis. J. Biol. Chem. 2006;281:19655–19664. - PubMed
V体育安卓版 - Publication types
V体育ios版 - MeSH terms
- VSports手机版 - Actions
- VSports最新版本 - Actions
- VSports app下载 - Actions
- "VSports" Actions
- Actions (V体育平台登录)
V体育2025版 - Substances
- "VSports在线直播" Actions
- V体育平台登录 - Actions
- "VSports最新版本" Actions
- Actions (VSports注册入口)
V体育安卓版 - LinkOut - more resources
Full Text Sources
Molecular Biology Databases (VSports app下载)
