Evaluation of systemic and mucosal anti-HPV16 and anti-HPV18 antibody responses from vaccinated women
- PMID: 18541349
- PMCID: PMC4131294
- DOI: 10.1016/j.vaccine.2008.04.074
"V体育平台登录" Evaluation of systemic and mucosal anti-HPV16 and anti-HPV18 antibody responses from vaccinated women
VSports app下载 - Abstract
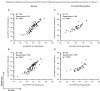
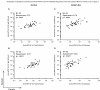
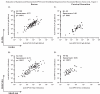
Ideal methods to monitor HPV neutralizing antibodies induced by vaccination have not been established yet. Here, we evaluated systemic and cervical antibody levels induced by HPV16/18 AS04-adjuvanted vaccine (GlaxoSmithKline Biologicals) using a secreted alkaline phosphatase neutralization assay (SEAP-NA) and enzyme-linked immunosorbent assay (ELISA). Serum and cervical secretions from 50 vaccinated women were used to assess (1) overall assay reproducibility; (2) inter-assay and inter-specimen correlation; (3) correlations between month 1 and month 12 titers. Strong correlations between SEAP-NA and ELISA were observed (serum anti-HPV16/18, rho=0. 91/0. 85; cervix anti-HPV16/18, rho=0. 84/0. 89) VSports手机版. Systemic and cervical antibody measures also correlated well (rho range: 0. 64-0. 75); except at mid-cycle (rho range: 0. 28-0. 65). Correlations between antibody levels at 1 and 12 months following the start of vaccination were poor (rho range: 0. 16-0. 38). In conclusion, HPV16/18 VLP-based ELISA is a reliable and valid method to monitor anti-HPV16/18 neutralizing potential for the first year following vaccination; however, additional studies will be required to better define the effects of the time on cycle and patterns of antibody response over time following vaccination. .
"VSports" Figures
"VSports最新版本" References
-
- Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–27. - PubMed
-
- Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6(4):204. - PubMed
-
- Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87(11):796–802. - PubMed
-
- Munoz N, Bosch FX, Castellsague X, Diaz M, de Sanjose S, Hammouda D, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111(2):278–85. - PubMed
-
- Castellsague X, Diaz M, de Sanjose S, Munoz N, Herrero R, Franceschi S, et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst. 2006;98(5):303–15. - PubMed
Publication types
- VSports注册入口 - Actions
MeSH terms
- Actions (VSports注册入口)
- V体育官网入口 - Actions
- VSports注册入口 - Actions
- V体育安卓版 - Actions
- "VSports最新版本" Actions
- "VSports手机版" Actions
- Actions (VSports)
- "VSports" Actions
Substances
- Actions (V体育安卓版)
V体育官网入口 - Grants and funding
LinkOut - more resources
"VSports" Full Text Sources
Other Literature Sources (V体育2025版)

