The E75 HER2/neu peptide vaccine (VSports app下载)
- PMID: 18536917
- PMCID: PMC11029853
- DOI: 10.1007/s00262-008-0540-3
The E75 HER2/neu peptide vaccine (V体育ios版)
Abstract
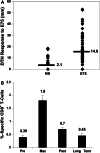
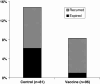
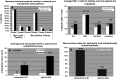
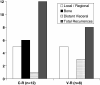
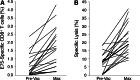
E75 (HER2/neu 369-377) is an immunogenic peptide from the HER2/neu protein which is overexpressed in many breast cancer patients. A large amount of preclinical work and a small number of Phase I trials have been completed evaluating the vaccine potential of the E75 peptide mixed with an immunoadjuvant. Our group has performed two concurrent E75 + GM-CSF Phase II trials in node-positive and node-negative disease-free breast cancer patients. These trials, totaling 186 patients, were designed to assess the ability of the E75 vaccine to prevent disease recurrence in these high risk patients. In this review article, we discuss the safety of the vaccine, the immunologic response to the peptide, and most importantly, the potential clinical benefit of the vaccine. The recurrence rate, mortality associated with recurrence, and the distribution of recurrences are presented and discussed. Additionally, the lessons learned from these trials to include optimal dosing and the need for booster inoculations are addressed VSports手机版. We also present data exploring possible explanations and mechanisms behind the potential clinical utility of this simple single epitope vaccine. Finally, we present some of the future directions for our Cancer Vaccine Development Program assessing multi-epitope peptide vaccines and combination immunotherapies. .
Figures
References
-
- Ko BK, Kawano K, Murray JL, Disis ML, Efferson CL, Kuerer HM, Peoples GE, Ioannides CG. Clinical studies of vaccines targeting breast cancer. Clin Cancer Res. 2003;9:3222–3234. - PubMed
Publication types
- "VSports注册入口" Actions
MeSH terms
- "VSports注册入口" Actions
- Actions (VSports在线直播)
- "V体育平台登录" Actions
- "V体育ios版" Actions
- "V体育官网" Actions
- Actions (V体育平台登录)
- VSports - Actions
Substances
- "V体育官网" Actions
"VSports注册入口" LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
V体育官网 - Research Materials
Miscellaneous

