Endothelial CD47 interaction with SIRPgamma is required for human T-cell transendothelial migration under shear flow conditions in vitro
- PMID: 18524990
- PMCID: PMC2515120
- DOI: 10.1182/blood-2008-01-134429
Endothelial CD47 interaction with SIRPgamma is required for human T-cell transendothelial migration under shear flow conditions in vitro
"V体育平台登录" Abstract
Leukocyte transendothelial migration (TEM) is a critical event during inflammation. CD47 has been implicated in myeloid cell migration across endothelium and epithelium. CD47 binds to signal regulatory protein (SIRP), SIRPalpha and SIRPgamma. So far, little is known about the role of endothelial CD47 in T-cell TEM in vivo or under flow conditions in vitro. Fluorescence-activated cell sorting and biochemical analysis show that CD3(+) T cells express SIRPgamma but not SIRPalpha, and fluorescence microscopy showed that CD47 was enriched at endothelial junctions. These expression patterns suggested that CD47 plays a role in T-cell TEM through binding interactions with SIRPgamma VSports手机版. We tested, therefore, whether CD47-SIRPgamma interactions affect T-cell transmigration using blocking mAb against CD47 or SIRPgamma in an in vitro flow model. These antibodies inhibited T-cell TEM by 70% plus or minus 6% and 82% plus or minus 1%, respectively, but had no effect on adhesion. In agreement with human mAb studies, transmigration of murine wild-type T helper type 1 cells across TNF-alpha-activated murine CD47(-/-) endothelium was reduced by 75% plus or minus 2% even though murine T cells appear to lack SIRPgamma. Nonetheless, these findings suggest endothelial cell CD47 interacting with T-cell ligands, such as SIRPgamma, play an important role in T-cell transendothelial migration. .
Figures
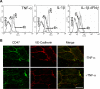

 ), B6H12 (middle, ▶), and control W6/32 (right) mAbs are shown. Arrows show migrated CD3+ cells in medium-treated monolayers. In contrast, T cells (▶) on B6H12-treated monolayers exhibit a rounded-up morphology and defects in migratory behavior. (Inset) A higher magnification of a single T cell during TEM is shown in the right upper corner of each DIC image to better show the differences in morphology in the presence or absence of a blocking CD47 antibody. (D) The migration velocity of T cells perfused on a HUVEC monolayer treated with B6H12 or W6/32 mAb or left untreated was determined using Image J software. *P = .01 versus W6/32 mAb.
), B6H12 (middle, ▶), and control W6/32 (right) mAbs are shown. Arrows show migrated CD3+ cells in medium-treated monolayers. In contrast, T cells (▶) on B6H12-treated monolayers exhibit a rounded-up morphology and defects in migratory behavior. (Inset) A higher magnification of a single T cell during TEM is shown in the right upper corner of each DIC image to better show the differences in morphology in the presence or absence of a blocking CD47 antibody. (D) The migration velocity of T cells perfused on a HUVEC monolayer treated with B6H12 or W6/32 mAb or left untreated was determined using Image J software. *P = .01 versus W6/32 mAb.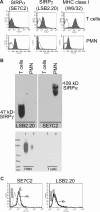
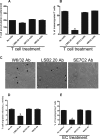
 show normal, migrated CD3+ cells in W6/32-treated monolayers. In contrast, T cells (▶) on SIRPγ-treated T cells exhibit a rounded-up morphology and defects in migratory behavior and show reduced transmigration. (D) The apical migration velocities of T cells on HUVEC monolayers treated with SIRPα (SE7C2), SIRPγ (LSB2.20), or nonblocking control mAb MHC class I mAbs was determined by the Image J software. *P = .01. (E) TNF-α–stimulated HUVEC monolayers were preincubated for 30 minutes with anti-CD47 (B6H12) or SIRPα (SE7C2) mAbs. Medium alone (no additions) or MHC-class I (W6/32) mAb served as controls. The percentage of T cells that transmigrated after 10 minutes was determined as described in “Methods.” Data are means (± SEM) of 4 separate experiments; *P = .001.
show normal, migrated CD3+ cells in W6/32-treated monolayers. In contrast, T cells (▶) on SIRPγ-treated T cells exhibit a rounded-up morphology and defects in migratory behavior and show reduced transmigration. (D) The apical migration velocities of T cells on HUVEC monolayers treated with SIRPα (SE7C2), SIRPγ (LSB2.20), or nonblocking control mAb MHC class I mAbs was determined by the Image J software. *P = .01. (E) TNF-α–stimulated HUVEC monolayers were preincubated for 30 minutes with anti-CD47 (B6H12) or SIRPα (SE7C2) mAbs. Medium alone (no additions) or MHC-class I (W6/32) mAb served as controls. The percentage of T cells that transmigrated after 10 minutes was determined as described in “Methods.” Data are means (± SEM) of 4 separate experiments; *P = .001.
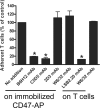
 identify migrated CD3+ cells (left), and ▶ show CD3+ cells with a rounded-up morphology and impaired apical migration on TNF-α stimulated CD47−/− MHEC (right). (E,F) WT and CD47−/− MHECs were stained for listed adhesion molecules in the presence (right) or absence (left) of TNF-α and analyzed by flow cytometry. Expression levels were shown in histograms and quantitated as mean fluorescence intensity. Data are means (± SD), n = 3 independent MHEC preparations; indicates statistical significant difference (P = .001).
identify migrated CD3+ cells (left), and ▶ show CD3+ cells with a rounded-up morphology and impaired apical migration on TNF-α stimulated CD47−/− MHEC (right). (E,F) WT and CD47−/− MHECs were stained for listed adhesion molecules in the presence (right) or absence (left) of TNF-α and analyzed by flow cytometry. Expression levels were shown in histograms and quantitated as mean fluorescence intensity. Data are means (± SD), n = 3 independent MHEC preparations; indicates statistical significant difference (P = .001).
References
-
- Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. - PubMed (V体育官网入口)
-
- Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res. 2007;101:234–247. - PubMed
-
- Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–135. - "VSports app下载" PubMed
-
- Barclay AN, Brown MH. The SIRP family of receptors and immune regulation. Nat Rev Immunol. 2006;6:457–464. - PubMed
Publication types
- Actions (VSports手机版)
- V体育官网入口 - Actions
MeSH terms (V体育2025版)
- Actions (V体育安卓版)
- Actions (VSports注册入口)
- VSports最新版本 - Actions
- "V体育平台登录" Actions
- "V体育官网" Actions
- VSports - Actions
- "VSports注册入口" Actions
- Actions (VSports)
Substances
- "VSports在线直播" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
V体育平台登录 - Molecular Biology Databases
Research Materials

