"VSports最新版本" A second pilus type in Streptococcus pneumoniae is prevalent in emerging serotypes and mediates adhesion to host cells
- PMID: 18515415
- PMCID: PMC2493256
- DOI: 10.1128/JB.00384-08 (V体育2025版)
V体育平台登录 - A second pilus type in Streptococcus pneumoniae is prevalent in emerging serotypes and mediates adhesion to host cells
Abstract
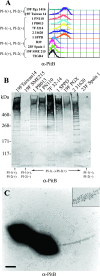
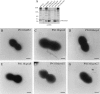
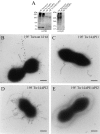
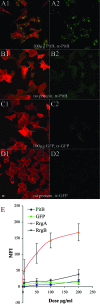
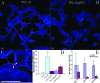
Analysis of publicly available genomes of Streptococcus pneumoniae has led to the identification of a new genomic element containing genes typical of gram-positive pilus islets (PIs). Here, we demonstrate that this genomic region, herein referred to as PI-2 (consisting of pitA, sipA, pitB, srtG1, and srtG2) codes for a second functional pilus in pneumococcus. Polymerization of the PI-2 pilus requires the backbone protein PitB as well as the sortase SrtG1 and the signal peptidase-like protein SipA. Presence of PI-2 correlates with the genotype as defined by multilocus sequence typing and clonal complex (CC). The PI-2-positive CCs are associated with serotypes 1, 2, 7F, 19A, and 19F, considered to be emerging serotypes in both industrialized and developing countries VSports手机版. Interestingly, strains belonging to CC271 (where sequence type 271 is the predicted founder of the CC) contain both PI-1 and PI-2, as revealed by genome analyses. In these strains both pili are surface exposed and independently assembled. Furthermore, in vitro experiments provide evidence that the pilus encoded by PI-2 of S. pneumoniae is involved in adherence. Thus, pneumococci encode at least two types of pili that play a role in the initial host cell contact to the respiratory tract and are potential antigens for inclusion in a new generation of pneumococcal vaccines. .
Figures

References
-
- Abbot, E. L., W. D. Smith, G. P. Siou, C. Chiriboga, R. J. Smith, J. A. Wilson, B. H. Hirst, and M. A. Kehoe. 2007. Pili mediate specific adhesion of Streptococcus pyogenes to human tonsil and skin. Cell Microbiol. 91822-1833. - "V体育官网" PubMed
-
- Alloing, G., B. Martin, C. Granadel, and J. P. Claverys. 1998. Development of competence in Streptococcus pneumonaie: pheromone autoinduction and control of quorum sensing by the oligopeptide permease. Mol. Microbiol. 2975-83. - PubMed
-
- Barocchi, M. A., J. Ries, X. Zogaj, C. Hemsley, B. Albiger, A. Kanth, S. Dahlberg, J. Fernebro, M. Moschioni, V. Masignani, K. Hultenby, A. R. Taddei, K. Beiter, F. Wartha, A. von Euler, A. Covacci, D. W. Holden, S. Normark, R. Rappuoli, and B. Henriques-Normark. 2006. A pneumococcal pilus influences virulence and host inflammatory responses. Proc. Natl. Acad. Sci. USA 1032857-2862. - PMC - PubMed
-
- Basset, A., K. Trzcinski, C. Hermos, L. O'Brien, K., R. Reid, M. Santosham, A. J. McAdam, M. Lipsitch, and R. Malley. 2007. Association of the pneumococcal pilus with certain capsular serotypes but not with increased virulence. J. Clin. Microbiol. 451684-1689. - PMC (VSports最新版本) - PubMed
VSports - MeSH terms
- "VSports注册入口" Actions
- V体育安卓版 - Actions
- VSports app下载 - Actions
- VSports在线直播 - Actions
- Actions (V体育官网)
- "V体育安卓版" Actions
- Actions (VSports手机版)
- V体育平台登录 - Actions
- Actions (V体育2025版)
- V体育平台登录 - Actions
Substances (VSports手机版)
"VSports" Associated data
- Actions (V体育ios版)
- Actions
- "VSports app下载" Actions
- VSports注册入口 - Actions
- V体育官网入口 - Actions
- "VSports最新版本" Actions
"VSports app下载" LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
VSports最新版本 - Miscellaneous

