Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin
- PMID: 18497879
- PMCID: PMC2391066
- DOI: 10.1172/JCI34584
Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin (VSports在线直播)
Abstract
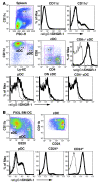
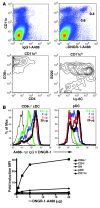
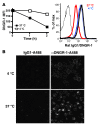
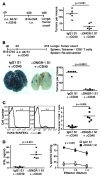
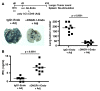
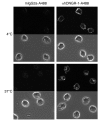
The mouse CD8alpha+ DC subset excels at cross-presentation of antigen, which can elicit robust CTL responses. A receptor allowing specific antigen targeting to this subset and its equivalent in humans would therefore be useful for the induction of antitumor CTLs. Here, we have characterized a C-type lectin of the NK cell receptor group that we named DC, NK lectin group receptor-1 (DNGR-1). DNGR-1 was found to be expressed in mice at high levels by CD8+ DCs and at low levels by plasmacytoid DCs but not by other hematopoietic cells. Human DNGR-1 was also restricted in expression to a small subset of blood DCs that bear similarities to mouse CD8alpha+ DCs. The selective expression pattern and observed endocytic activity of DNGR-1 suggested that it could be used for antigen targeting to DCs. Consistent with this notion, antigen epitopes covalently coupled to an antibody specific for mouse DNGR-1 were selectively cross-presented by CD8alpha+ DCs in vivo and, when given with adjuvants, induced potent CTL responses. When the antigens corresponded to tumor-expressed peptides, treatment with the antibody conjugate and adjuvant could prevent development or mediate eradication of B16 melanoma lung pseudometastases. We conclude that DNGR-1 is a novel, highly specific marker of mouse and human DC subsets that can be exploited for CTL cross-priming and tumor therapy. VSports手机版.
"VSports app下载" Figures

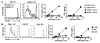


References
-
- Steinman R. Dendritic cells: versatile controllers of the immune system. Nat. Med. 2007;13:1155–1159. doi: 10.1038/nm1643. - "VSports最新版本" DOI - PubMed
-
- Vremec D., Shortman K. Dendritic cell subtypes in mouse lymphoid organs: cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J. Immunol. 1997;159:565–573. - PubMed
-
- Henri S., et al. The dendritic cell populations of mouse lymph nodes. J. Immunol. 2001;167:741–748. - VSports注册入口 - PubMed
Publication types
- Actions (VSports注册入口)
MeSH terms
- "VSports app下载" Actions
- Actions (V体育2025版)
- VSports注册入口 - Actions
- VSports在线直播 - Actions
- "VSports注册入口" Actions
- VSports在线直播 - Actions
- "V体育2025版" Actions
- "VSports app下载" Actions
- Actions (V体育平台登录)
- VSports手机版 - Actions
- "VSports注册入口" Actions
- "V体育安卓版" Actions
- "V体育ios版" Actions
VSports - Substances
- "V体育平台登录" Actions
- V体育官网 - Actions
- "V体育安卓版" Actions
Grants and funding
"V体育安卓版" LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
"V体育官网入口" Research Materials

