V体育ios版 - Control of hematopoietic stem cell quiescence by the E3 ubiquitin ligase Fbw7
- PMID: 18474632
- PMCID: PMC2413036
- DOI: "V体育平台登录" 10.1084/jem.20080277
Control of hematopoietic stem cell quiescence by the E3 ubiquitin ligase Fbw7
Abstract
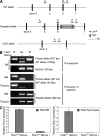
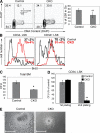
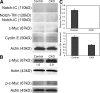
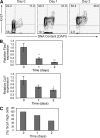
Ubiquitination is a posttranslational mechanism that controls diverse cellular processes. We focus here on the ubiquitin ligase Fbw7, a recently identified hematopoietic tumor suppressor that can target for degradation several important oncogenes, including Notch1, c-Myc, and cyclin E. We have generated conditional Fbw7 knockout animals and inactivated the gene in hematopoietic stem cells (HSCs), progenitors, and their differentiated progeny. Deletion of Fbw7 specifically and rapidly affects hematopoiesis in a cell-autonomous manner. Fbw7(-/-) HSCs show defective maintenance of quiescence, leading to impaired self-renewal and a severe loss of competitive repopulating capacity. Furthermore, Fbw7(-/-) progenitors are unable to colonize the thymus, leading to a profound depletion of T cell progenitors. Deletion of Fbw7 in bone marrow (BM) stem cells and progenitors leads to the stabilization of c-Myc, a transcription factor previously implicated in HSC self-renewal VSports手机版. On the other hand, neither Notch1 nor cyclin E is visibly stabilized in the BM of Fbw7-deficient mice. Gene expression studies of Fbw7(-/-) HSCs and hematopoietic progenitors indicate that Fbw7 regulates, through the regulation of HSC cycle entry, the transcriptional "signature" that is associated with the quiescent, self-renewing HSC phenotype. .
Figures



References (VSports注册入口)
-
- Passegue, E., and A.J. Wagers. 2006. Regulating quiescence: new insights into hematopoietic stem cell biology. Dev. Cell. 10:415–417. - VSports - PubMed
-
- Aguila, H.L., K. Akashi, J. Domen, K.L. Gandy, E. Lagasse, R.E. Mebius, S.J. Morrison, J. Shizuru, S. Strober, N. Uchida, et al. 1997. From stem cells to lymphocytes: biology and transplantation. Immunol. Rev. 157:13–40. - "VSports最新版本" PubMed
-
- Adams, G.B., and D.T. Scadden. 2006. The hematopoietic stem cell in its place. Nat. Immunol. 7:333–337. - PubMed
Publication types
- "V体育安卓版" Actions
V体育ios版 - MeSH terms
- Actions (V体育2025版)
- "V体育ios版" Actions
- VSports注册入口 - Actions
- Actions (V体育官网入口)
- V体育官网入口 - Actions
- "V体育2025版" Actions
- V体育官网 - Actions
- "VSports最新版本" Actions
- "VSports注册入口" Actions
- Actions (VSports注册入口)
- "VSports手机版" Actions
- Actions (VSports)
Substances
- "V体育2025版" Actions
- VSports app下载 - Actions
- "V体育2025版" Actions
V体育官网入口 - Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
"V体育平台登录" Medical
Molecular Biology Databases
"VSports在线直播" Research Materials

