VSports手机版 - Persistence of repair proteins at unrepaired DNA damage distinguishes diseases with ERCC2 (XPD) mutations: cancer-prone xeroderma pigmentosum vs. non-cancer-prone trichothiodystrophy
- PMID: 18470933
- PMCID: PMC3477783
- DOI: 10.1002/humu.20768
Persistence of repair proteins at unrepaired DNA damage distinguishes diseases with ERCC2 (XPD) mutations: cancer-prone xeroderma pigmentosum vs. non-cancer-prone trichothiodystrophy
Abstract (V体育2025版)
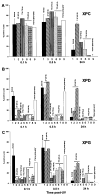
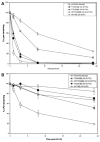
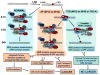
Patients with xeroderma pigmentosum (XP) have a 1,000-fold increase in ultraviolet (UV)-induced skin cancers while trichothiodystrophy (TTD) patients, despite mutations in the same genes, ERCC2 (XPD) or ERCC3 (XPB), are cancer-free. Unlike XP cells, TTD cells have a nearly normal rate of removal of UV-induced 6-4 photoproducts (6-4PP) in their DNA and low levels of the basal transcription factor, TFIIH. We examined seven XP, TTD, and XP/TTD complex patients and identified mutations in the XPD gene VSports手机版. We discovered large differences in nucleotide excision repair (NER) protein recruitment to sites of localized UV damage in TTD cells compared to XP or normal cells. XPC protein was rapidly localized in all cells. XPC was redistributed in TTD, and normal cells by 3 hr postirradiation, but remained localized in XP cells at 24-hr postirradiation. In XP cells recruitment of other NER proteins (XPB, XPD, XPG, XPA, and XPF) was also delayed and persisted at 24 hr (p<0. 001). In TTD cells with defects in the XPD, XPB, or GTF2H5 (TTDA) genes, in contrast, recruitment of these NER proteins was reduced compared to normals at early time points (p<0. 001) and remained low at 24 hr postirradiation. These data indicate that in XP persistence of NER proteins at sites of unrepaired DNA damage is associated with greatly increased skin cancer risk possibly by blockage of translesion DNA synthesis. In contrast, in TTD, low levels of unstable TFIIH proteins do not accumulate at sites of unrepaired photoproducts and may permit normal translesion DNA synthesis without increased skin cancer. .
Figures




References
-
- Ahrens C, Grewe M, Berneburg M, Grether-Beck S, Quilliet X, Mezzina M, Sarasin A, Lehmann AR, Arlett CF, Krutmann J. Photocarcinogenesis and inhibition of intercellular adhesion molecule 1 expression in cells of DNA-repair-defective individuals. Proc Natl Acad Sci USA. 1997;94:6837–6841. - PMC - PubMed
-
- Andrews AD, Barrett SF, Robbins JH. Xeroderma pigmentosum neurological abnormalities correlate with colony-forming ability after ultraviolet radiation. Proc Natl Acad Sci USA. 1978;75:1984–1988. - PMC (V体育官网入口) - PubMed
-
- Barrett SF, Robbins JH, Tarone RE, Kraemer KH. Evidence for defective repair of cyclobutane pyrimidine dimers with normal repair of other DNA photoproducts in a transcriptionally active gene transfected into Cockayne syndrome cells. Mutat Res. 1991;255:281–291. - PubMed
-
- Berneburg M, Clingen PH, Harcourt SA, Lowe JE, Taylor EM, Green MH, Krutmann J, Arlett CF, Lehmann AR. The cancer-free phenotype in trichothiodystrophy is unrelated to its repair defect. Cancer Res. 2000;60:431–438. - PubMed
-
- Bootsma D, Hoeijmakers JH. DNA repair. Engagement with transcription. Nature. 1993;363:114–115. - PubMed
MeSH terms
- "V体育2025版" Actions
- Actions (VSports)
- VSports注册入口 - Actions
- VSports app下载 - Actions
- "V体育安卓版" Actions
- VSports - Actions
- Actions (VSports)
- V体育ios版 - Actions
- "VSports手机版" Actions
- Actions (V体育官网)
- "V体育官网" Actions
Substances
- Actions (VSports)
- Actions (V体育ios版)
- V体育安卓版 - Actions
- "VSports注册入口" Actions
- V体育安卓版 - Actions
- "V体育安卓版" Actions
- "V体育ios版" Actions
- "VSports最新版本" Actions
- V体育安卓版 - Actions
Grants and funding
"V体育官网" LinkOut - more resources
Full Text Sources
Medical
Research Materials

