VSports app下载 - Akt3 and mutant V600E B-Raf cooperate to promote early melanoma development
- PMID: 18451171
- PMCID: PMC2603082
- DOI: "V体育平台登录" 10.1158/0008-5472.CAN-07-5867
Akt3 and mutant V600E B-Raf cooperate to promote early melanoma development
Abstract
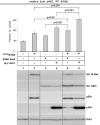
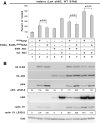
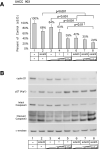
B-Raf is the most mutated gene in melanoma; however, the mechanism through which it promotes early melanomas remains uncertain. Most nevi contain activated (V600E)B-Raf but few develop into melanoma, and expression in melanocytes is inhibitory with low protein levels present in surviving cells, suggesting unknown cooperative oncogenic events are necessary for melanoma development VSports手机版. Because many melanomas have (V600E)B-Raf and active Akt3, it is possible that these proteins cooperatively facilitate melanocyte transformation. In this study, Akt3 is shown to phosphorylate (V600E)B-Raf to lower its activity as well as that of the downstream mitogen-activated protein kinase (MAPK) pathway to levels promoting early melanoma development. Expression of active Akt3 in early melanoma cells containing (V600E)B-Raf reduced MAPK signaling and promoted anchorage-independent growth. Furthermore, expression of both (V600E)B-Raf and active Akt3 in melanocytes promoted a transformed phenotype. Mechanistically, aberrant Akt3 activity in early melanomas serves to phosphorylate Ser(364) and Ser(428) on (V600E)B-Raf to reduce activity of (V600E)B-Raf to levels that promote rather than inhibit proliferation, which aids melanocytic transformation. Inhibition of (V600E)B-Raf or Akt3 in advanced melanoma cells in which both pathways were active reduced anchorage-independent growth and tumor development in a cooperatively acting manner. Inhibition of Akt3 alone in these cells led to increased MAPK signaling. In summary, these results suggest that activating B-Raf mutations initially promote nevi development, but the resulting high, intense activation of the MAPK pathway inhibits further tumor progression requiring Akt3 activation to bypass this barrier and aid melanoma development. .
Figures











References
-
- Cancer Facts and Figures. American Cancer Society; Atlanta: 2005.
-
- Smalley KS, Herlyn M. Targeting intracellular signaling pathways as a novel strategy in melanoma therapeutics. Ann N Y Acad Sci. 2005;1059:16–25. - PubMed (VSports app下载)
-
- Hsu MY, Meier F, Herlyn M. Melanoma development and progression: a conspiracy between tumor and host. Differentiation. 2002;70:522–36. - PubMed
Publication types
- "V体育官网" Actions
MeSH terms
- V体育平台登录 - Actions
- V体育官网入口 - Actions
- Actions (VSports注册入口)
- "VSports" Actions
- "V体育官网" Actions
- Actions (VSports最新版本)
- Actions (VSports最新版本)
- Actions (VSports注册入口)
- "VSports app下载" Actions
- Actions (V体育2025版)
- Actions (V体育平台登录)
- V体育2025版 - Actions
- Actions (V体育官网入口)
- V体育平台登录 - Actions
Substances (VSports在线直播)
- Actions (VSports)
- "VSports app下载" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials (V体育ios版)
Miscellaneous (V体育ios版)

