The human CXC chemokine granulocyte chemotactic protein 2 (GCP-2)/CXCL6 possesses membrane-disrupting properties and is antibacterial
- PMID: 18443119
- PMCID: PMC2443903 (VSports在线直播)
- DOI: 10.1128/AAC.00028-08
V体育平台登录 - The human CXC chemokine granulocyte chemotactic protein 2 (GCP-2)/CXCL6 possesses membrane-disrupting properties and is antibacterial
Abstract
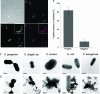
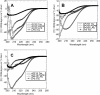
Granulocyte chemotactic protein 2 (GCP-2)/CXCL6 is a CXC chemokine expressed by macrophages and epithelial and mesenchymal cells during inflammation. Through binding and activation of its receptors (CXCR1 and CXCR2), it exerts neutrophil-activating and angiogenic activities. Here we show that GCP-2/CXCL6 itself is antibacterial. Antibacterial activity against gram-positive and gram-negative pathogenic bacteria of relevance to mucosal infections was seen at submicromolar concentrations (minimal bactericidal concentration at which 50% of strains tested were killed, 0 VSports手机版. 063 +/- 0. 01 to 0. 37 +/- 0. 03 muM). In killed bacteria, GCP-2/CXCL6 associated with bacterial surfaces, which showed membrane disruption and leakage. A structural prediction indicated the presence of three antiparallel NH(2)-terminal beta-sheets and a short amphipathic COOH-terminal alpha-helix; the latter feature is typical of antimicrobial peptides. However, when the synthetic derivatives corresponding to the NH(2)-terminal (50 amino acids) and COOH-terminal (19 amino acids, corresponding to the putative alpha-helix) regions were compared, higher antibacterial activity was observed for the NH(2)-terminus-derived peptide, indicating that the holopeptide is necessary for full antibacterial activity. An artificial model of bacterial membranes confirmed these findings. The helical content of GCP-2/CXCL6 in the presence or absence of lipopolysaccharide or negatively charged membranes was studied by circular dichroism. As with many antibacterial peptides, membrane disruption by GCP-2/CXCL6 was dose-dependently reduced in the presence of NaCl, which, we here demonstrate, inhibited the binding of the peptide to the bacterial surface. Compared with CXC chemokines ENA-78/CXCL5 and NAP-2/CXCL7, GCP-2/CXCL6 showed a 90-fold-higher antibacterial activity. Taken together, GCP/CXCL6, in addition to its chemotactic and angiogenic properties, is likely to contribute to direct antibacterial activity during localized infection. .
Figures


References
-
- Baggiolini, M. 2001. Chemokines in pathology and medicine. J. Intern. Med. 250:91-104. - V体育平台登录 - PubMed
-
- Boman, H. G. 2003. Antibacterial peptides: basic facts and emerging concepts. J. Intern. Med. 254:197-215. - PubMed
-
- Chertov, O., D. F. Michiel, L. Xu, J. M. Wang, K. Tani, W. J. Murphy, D. L. Longo, D. D. Taub, and J. J. Oppenheim. 1996. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J. Biol. Chem. 271:2935-2940. - PubMed
-
- Cole, A. M., T. Ganz, A. M. Liese, M. D. Burdick, L. Liu, and R. M. Strieter. 2001. IFN-inducible ELR-CXC chemokines display defensin-like antimicrobial activity. J. Immunol. 167:623-627. - "V体育官网入口" PubMed
-
- Egesten, A., M. Eliasson, H. M. Johansson, A. I. Olin, M. Mörgelin, A. Mueller, J. E. Pease, I. M. Frick, and L. Björck. 2007. The CXC chemokine MIG/CXCL9 is important in innate immunity against Streptococcus pyogenes. J. Infect. Dis. 195:684-693. - PubMed
"V体育ios版" Publication types
MeSH terms
- Actions (VSports app下载)
- "VSports app下载" Actions
- "V体育ios版" Actions
- "VSports app下载" Actions
- "V体育官网" Actions
- VSports手机版 - Actions
- "V体育安卓版" Actions
- "VSports注册入口" Actions
- "VSports app下载" Actions
- "V体育平台登录" Actions
- Actions (VSports最新版本)
- "VSports在线直播" Actions
- Actions (VSports注册入口)
- Actions (V体育官网入口)
- "V体育安卓版" Actions
- "VSports注册入口" Actions
Substances
- "VSports手机版" Actions
- "VSports" Actions
- VSports最新版本 - Actions
VSports最新版本 - LinkOut - more resources
"VSports注册入口" Full Text Sources
Medical
Molecular Biology Databases
Research Materials (VSports手机版)

