Dexamethasone transforms lipopolysaccharide-stimulated human blood myeloid dendritic cells into myeloid dendritic cells that prime interleukin-10 production in T cells
- PMID: 18312359
- PMCID: PMC2526263
- DOI: 10.1111/j.1365-2567.2008.02824.x
Dexamethasone transforms lipopolysaccharide-stimulated human blood myeloid dendritic cells into myeloid dendritic cells that prime interleukin-10 production in T cells (V体育2025版)
Abstract
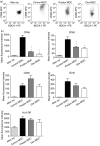
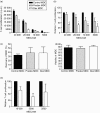
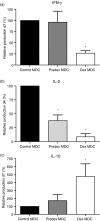
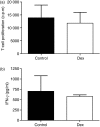
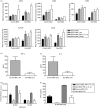
Myeloid dendritic cells (MDC) play an important role in antigen-specific immunity and tolerance. In transplantation setting donor-derived MDC are a promising tool to realize donor-specific tolerance. Current protocols enable generation of tolerogenic donor MDC from human monocytes during 1-week cultures. However, for clinical application in transplantation medicine, a rapidly available source of tolerogenic MDC is desired. In this study we investigated whether primary human blood MDC could be transformed into tolerogenic MDC using dexamethasone (dex) and lipopolysaccharide (LPS). Human blood MDC were cultured with dex and subsequently matured with LPS in the presence or absence of dex. Activation of MDC with LPS after pretreatment with dex did not prevent maturation into immunostimulatory MDC VSports手机版. In contrast, simultaneous treatment with dex and LPS yielded tolerogenic MDC, that had a reduced expression of CD86 and CD83, that poorly stimulated allogeneic T-cell proliferation and production of T helper 1 (Th1) cytokines, and primed production of the immunoregulatory cytokine interleukin-10 (IL-10) in T cells. In vitro, however, these tolerogenic MDC did not induce permanent donor-specific hyporesponsiveness in T cells. Importantly, tolerogenic MDC obtained by LPS stimulation in the presence of dex did not convert into immunostimulatory MDC after subsequent activation with different maturation stimuli. In conclusion, these findings demonstrate that combined treatment with dex and LPS transforms primary human blood MDC into tolerogenic MDC that are impaired to stimulate Th1 cytokines, but strongly prime the production of the immunoregulatory cytokine IL-10 in T cells, and are resistant to maturation stimuli. This strategy enables rapid generation of tolerogenic donor-derived MDC for immunotherapy in clinical transplantation. .
Figures
References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. - PubMed
-
- Lutz MB, Suri RM, Niimi M, Ogilvie AL, Kukutsch NA, Rossner S, Schuler G, Austyn JM. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur J Immunol. 2000;30:1813–22. - PubMed
-
- Morelli AE, Thomson AW. Dendritic cells: regulators of alloimmunity and opportunities for tolerance induction. Immunol Rev. 2003;196:125–46. - PubMed (VSports注册入口)
-
- Boumpas DT, Chrousos GP, Wilder RL, Cupps TR, Balow JE. Glucocorticoid therapy for immune-mediated diseases: basic and clinical correlates. Ann Intern Med. 1993;119:1198–208. - PubMed
MeSH terms
- VSports注册入口 - Actions
- "VSports最新版本" Actions
- V体育官网 - Actions
- Actions (VSports最新版本)
- VSports在线直播 - Actions
- "V体育官网" Actions
- Actions (VSports最新版本)
- "V体育官网" Actions
- Actions (VSports app下载)
- Actions (V体育官网入口)
VSports最新版本 - Substances
- Actions (V体育官网)
- V体育2025版 - Actions
- "V体育2025版" Actions
V体育官网 - LinkOut - more resources
Full Text Sources
V体育安卓版 - Medical

