A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy
- PMID: 18296641
- PMCID: PMC2265142
- DOI: "VSports" 10.1073/pnas.0712145105
A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy
Abstract
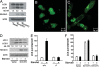
We demonstrate a role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. In particular, transient increased expression of Sirt1 is sufficient to stimulate basal rates of autophagy. In addition, we show that Sirt1(-/-) mouse embryonic fibroblasts do not fully activate autophagy under starved conditions. Reconstitution with wild-type but not a deacetylase-inactive mutant of Sirt1 restores autophagy in these cells VSports手机版. We further demonstrate that Sirt1 can form a molecular complex with several essential components of the autophagy machinery, including autophagy genes (Atg)5, Atg7, and Atg8. In vitro, Sirt1 can, in an NAD-dependent fashion, directly deacetylate these components. The absence of Sirt1 leads to markedly elevated acetylation of proteins known to be required for autophagy in both cultured cells and in embryonic and neonatal tissues. Finally, we show that Sirt1(-/-) mice partially resemble Atg5(-/-) mice, including the accumulation of damaged organelles, disruption of energy homeostasis, and early perinatal mortality. Furthermore, the in utero delivery of the metabolic substrate pyruvate extends the survival of Sirt1(-/-) pups. These results suggest that the Sirt1 deacetylase is an important in vivo regulator of autophagy and provide a link between sirtuin function and the overall cellular response to limited nutrients. .
VSports app下载 - Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ohsumi Y. Molecular dissection of autophagy: Two ubiquitin-like systems. Nat Rev Mol Cell Biol. 2001;2:211–216. - PubMed
-
- Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: Cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–448. - PubMed
-
- Kuma A, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. - PubMed
-
- Hara T, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. - PubMed
Publication types
- V体育官网入口 - Actions
MeSH terms
- "V体育2025版" Actions
- VSports注册入口 - Actions
- V体育平台登录 - Actions
- "VSports在线直播" Actions
- V体育ios版 - Actions
- "VSports注册入口" Actions
- VSports在线直播 - Actions
Substances
- "VSports手机版" Actions
- VSports最新版本 - Actions
- V体育2025版 - Actions
- "V体育官网" Actions
- Actions (VSports最新版本)
- V体育ios版 - Actions
- "VSports在线直播" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases (V体育ios版)
Research Materials

