Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis (VSports)
- PMID: 18188453
- PMCID: PMC2176188
- DOI: 10.1172/JCI33145 (V体育官网入口)
Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis
Abstract
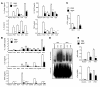
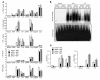
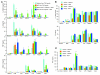
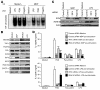
The mechanisms underlying the susceptibility of individuals with caspase recruitment domain 15 (CARD15) mutations and corresponding abnormalities of nucleotide-binding oligomerization domain 2 (NOD2) protein to Crohn disease are still poorly understood. One possibility is based on previous studies showing that muramyl dipeptide (MDP) activation of NOD2 negatively regulates TLR2 responses and that absence of such regulation leads to heightened Th1 responses. We now report that administration of MDP protects mice from the development of experimental colitis by downregulating multiple TLR responses, not just TLR2. The basis of these in vivo findings was suggested by in vitro studies of DCs, in which we showed that prestimulation of cells with MDP reduces cytokine responses to multiple TLR ligands and this reduction is dependent on enhanced IFN regulatory factor 4 (IRF4) activity. Further studies of mouse models of colitis showed that this inhibitory role of IRF4 does in fact apply to MDP-mediated protection from colitis, since neither IRF4-deficient mice nor mice treated with siRNA specific for IRF4 were protected. These findings indicate that MDP activation of NOD2 regulates innate responses to intestinal microflora by downregulating multiple TLR responses and suggest that the absence of such regulation leads to increased susceptibility to Crohn disease. VSports手机版.
Figures

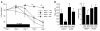
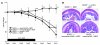



References (VSports最新版本)
-
- Strober W., Murray P.J., Kitani A., Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat. Rev. Immunol. 2006;6:9–20. - PubMed
-
- Inohara Chamaillard, onald C., Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu. Rev. Biochem. 2005;74:355–383. - PubMed
-
- Hugot J.P., et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. - PubMed
-
- Ogura Y., et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. - PubMed
V体育官网入口 - Publication types
- "VSports" Actions
MeSH terms
- "V体育安卓版" Actions
- V体育ios版 - Actions
- VSports最新版本 - Actions
- V体育2025版 - Actions
- VSports app下载 - Actions
- "VSports注册入口" Actions
- "VSports在线直播" Actions
- VSports - Actions
- VSports在线直播 - Actions
- "VSports注册入口" Actions
- V体育安卓版 - Actions
- Actions (VSports app下载)
- Actions (V体育2025版)
- V体育官网 - Actions
Substances
- VSports在线直播 - Actions
- Actions (V体育ios版)
- "V体育2025版" Actions
- "VSports app下载" Actions
- VSports - Actions
LinkOut - more resources
Full Text Sources
V体育官网 - Other Literature Sources
Medical
Molecular Biology Databases

