"VSports最新版本" The endogenous danger signal, crystalline uric acid, signals for enhanced antibody immunity
- PMID: 18029553
- PMCID: VSports手机版 - PMC2214762
- DOI: 10.1182/blood-2007-10-117184
The endogenous danger signal, crystalline uric acid, signals for enhanced antibody immunity
Abstract
Studies have shown that the immune system can recognize self-antigens under conditions (eg, cell injury) in which the self-tissue might elaborate immune-activating endogenous danger signals. Uric acid (UA) is an endogenous danger signal recently identified to be released from dying cells. Prior work has shown that UA activates immune effectors of both the innate and adaptive immune system, including neutrophils and cytotoxic T-cell immunity. However, it was unclear whether UA could enhance antibody immunity, which was examined in this study. When added to dying tumor cells or with whole protein antigen, UA increased IgG1-based humoral immunity. Further, UA blocked growth of tumor in subsequent tumor challenge experiments, which depended on CD4, but not CD8, T cells. Sera derived from UA-treated animals enhanced tumor growth, suggesting it had little role in the antitumor response. UA did not signal for T-cell expansion or altered tumor-infiltrating leukocyte populations VSports手机版. Consistent with the lack of T-cell expansion, when applied to dendritic cells, UA suppressed T-cell growth factors but up-regulated B cell-activating cytokines. Understanding the nature of endogenous danger signals released from dying cells may aid in a better understanding of mechanisms of immune recognition of self. .
V体育ios版 - Figures

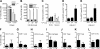
 ), and 25 μg UA alone (
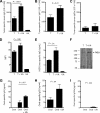
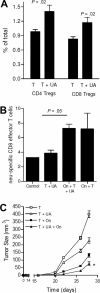
), and 25 μg UA alone ( ). Each bar represents the mean (± SEM) of 12 determinations. (B) The levels of IFN-γ–secreting T cells responding to no antigen, ConA, or neu-derived MHC class I peptide p420-429. Splenocytes were derived from animals that received dying tumor cells with various levels of UA. Each bar is the mean of 9 determinations. (C) The numbers of neu peptide p420-429 tetramer-positive T cells in draining nodes or splenocytes from mice depicted in panel F. Each is the mean (± SEM) of triplicate determinations calculated from 3 mice. (D,E) The frequencies of Oval(323)-specific CD4+DO11.10 T cells (D) and Oval(257)-specific CD8+OT-I T cells (E) as percentage of all CD3+ splenocytes in untreated control (Cont), ovalbumin cognate peptide/UA (UA), or cognate ovalbumin peptide/GM-CSF (GM). Each bar represents the mean (± SEM) of 7 replicates and represents 2 identical experiments, which yielded similar results. (F-L) The levels of various leukocytes in tumors from mice that received either no treatment (Cont) or pretreatment with tumor cells and 25 μg UA (T + UA), prior to tumor injection. Each bar is the mean (± SEM) of 3 replicates. Each graph represents a unique intratumoral leukocyte population from control, untreated mice and from mice pretreated with dying tumor cells containing UA. All are calculated from 100 000 total events. Results are expressed as the percentage of total cells, both tumor cells and leukocytes, collected following tumor mincing.
). Each bar represents the mean (± SEM) of 12 determinations. (B) The levels of IFN-γ–secreting T cells responding to no antigen, ConA, or neu-derived MHC class I peptide p420-429. Splenocytes were derived from animals that received dying tumor cells with various levels of UA. Each bar is the mean of 9 determinations. (C) The numbers of neu peptide p420-429 tetramer-positive T cells in draining nodes or splenocytes from mice depicted in panel F. Each is the mean (± SEM) of triplicate determinations calculated from 3 mice. (D,E) The frequencies of Oval(323)-specific CD4+DO11.10 T cells (D) and Oval(257)-specific CD8+OT-I T cells (E) as percentage of all CD3+ splenocytes in untreated control (Cont), ovalbumin cognate peptide/UA (UA), or cognate ovalbumin peptide/GM-CSF (GM). Each bar represents the mean (± SEM) of 7 replicates and represents 2 identical experiments, which yielded similar results. (F-L) The levels of various leukocytes in tumors from mice that received either no treatment (Cont) or pretreatment with tumor cells and 25 μg UA (T + UA), prior to tumor injection. Each bar is the mean (± SEM) of 3 replicates. Each graph represents a unique intratumoral leukocyte population from control, untreated mice and from mice pretreated with dying tumor cells containing UA. All are calculated from 100 000 total events. Results are expressed as the percentage of total cells, both tumor cells and leukocytes, collected following tumor mincing.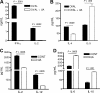

References
-
- Janeway CA., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–16. - PubMed
-
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. - PubMed
-
- Uematsu S, Akira S. Toll-like receptors and type I interferons. J Biol Chem. 2007;282:15319–15323. - "V体育平台登录" PubMed
-
- Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. - PubMed
Publication types
- V体育2025版 - Actions
MeSH terms
- "VSports注册入口" Actions
- V体育安卓版 - Actions
- "VSports" Actions
Substances
- "VSports最新版本" Actions
Grants and funding
LinkOut - more resources
"VSports最新版本" Full Text Sources
Research Materials

