Multicenter comparison of different real-time PCR assays for quantitative detection of Epstein-Barr virus
- PMID: 17989187
- PMCID: PMC2224294
- DOI: 10.1128/JCM.01252-07
Multicenter comparison of different real-time PCR assays for quantitative detection of Epstein-Barr virus
"VSports手机版" Abstract
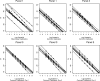
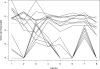
Quantification of Epstein-Barr virus (EBV) in peripheral blood is important for the diagnosis and management of serious EBV diseases, including posttransplant lymphoproliferative disorder. A variety of PCR-based methods are currently in use; however, there is little information on their comparability VSports手机版. This study assessed the relative performance of different quantitative assays. A multicenter comparative study was performed at eight sites using three panels consisting of serial dilutions of quantified EBV DNA and extracts from a total of 19 whole-blood specimens. Samples were distributed and tested blindly. Instrumentation, probe chemistries, amplification targets, and other test-related aspects varied considerably between laboratories. Each laboratory's calibration curve indicated strong evidence of a consistent log-linear relationship between viral load and cycle threshold, suggesting that intralaboratory tracking of a given patient would yield similar relative quantitative trends among the participating test sites. There was strong concordance among laboratories with respect to qualitative test results; however, marked quantitative discordance was seen. For most samples, the across-laboratory interquartile range of the reported viral load (in copies/microl) was roughly 0. 6 log-units, and for one sample the overall range was approximately 4. 2 log-units. While intralaboratory tracking of patients may yield similar results, these data indicate a need for caution when attempting to compare clinical results obtained at different institutions and suggest the potential value to be gained by more standardized testing methodology. .
Figures


References
-
- Bakker, N. A., E. A. Verschuuren, M. E. Erasmus, B. G. Hepkema, N. J. Veeger, C. G. Kallenberg, and W. van der Bij. 2007. Epstein-Barr virus-DNA load monitoring late after lung transplantation: a surrogate marker of the degree of immunosuppression and a safe guide to reduce immunosuppression. Transplantation 83433-438. - PubMed
-
- Cohen, J. I. 2005. Clinical aspects of Epstein-Barr virus infection, p. 35-54. In E. Robertson (ed.), Epstein-Barr virus. Caister Academic Press, Norfolk, England.
-
- Hammer, S. M., M. S. Saag, M. Schechter, J. S. Montaner, R. T. Schooley, D. M. Jacobsen, M. A. Thompson, C. C. Carpenter, M. A. Fischl, B. G. Gazzard, J. M. Gatell, M. S. Hirsch, D. A. Katzenstein, D. D. Richman, S. Vella, P. G. Yeni, and P. A. Volberding. 2006. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. Top. HIV Med. 14827-843. - PubMed
-
- Holmes, H., C. Davis, A. Heath, I. Hewlett, and N. Lelie. 2001. An international collaborative study to establish the first international standard for HIV-1 RNA for use in nucleic acid-based techniques. J. Virol. Methods 92141-150. - PubMed
Publication types
MeSH terms
- V体育官网 - Actions
- Actions (VSports app下载)
- "VSports注册入口" Actions
- V体育安卓版 - Actions
- V体育官网 - Actions
- VSports在线直播 - Actions
- "VSports在线直播" Actions
- Actions (VSports app下载)
Substances (VSports app下载)
LinkOut - more resources
Full Text Sources

