Photodynamic therapy-generated vaccines: relevance of tumour cell death expression (V体育安卓版)
- PMID: 17971767
- PMCID: "V体育安卓版" PMC2360230
- DOI: 10.1038/sj.bjc.6604059 (VSports手机版)
Photodynamic therapy-generated vaccines: relevance of tumour cell death expression
Abstract
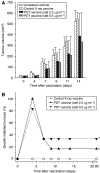
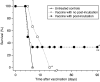
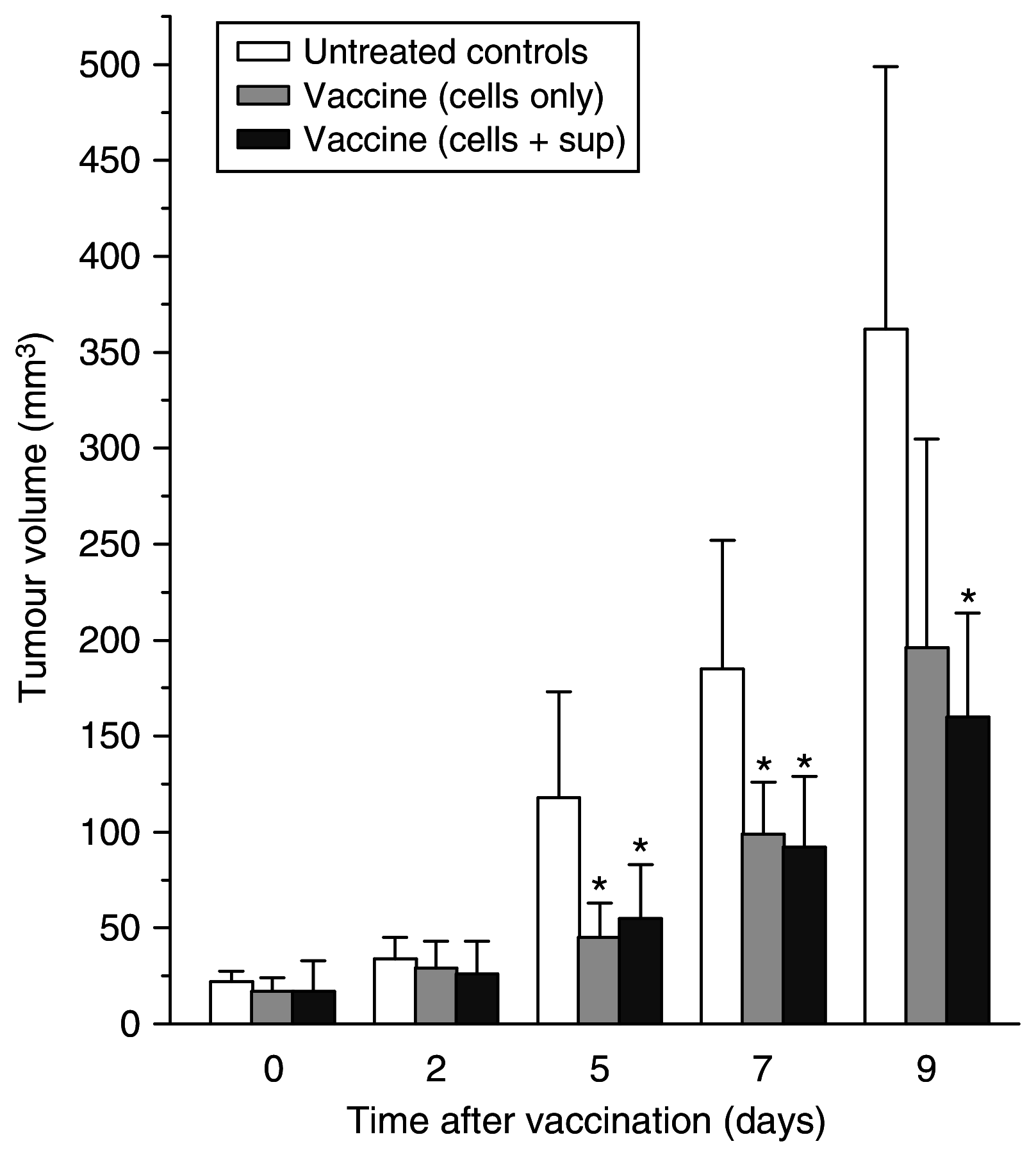
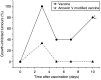
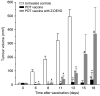
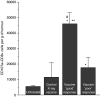
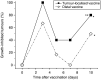
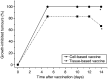
Recent investigations have established that tumour cells treated in vitro by photodynamic therapy (PDT) can be used for generating potent vaccines against cancers of the same origin. In the present study, cancer vaccines were prepared by treating mouse SCCVII squamous cell carcinoma cells with photosensitiser chlorin e6-based PDT and used against poorly immunogenic SCCVII tumours growing in syngeneic immunocompetent mice. The vaccine potency increased when cells were post-incubated in culture after PDT treatment for 16 h before they were injected into tumour-bearing mice. Interfering with surface expression of phosphatidylserine (annexin V treatment) and apoptosis (caspase inhibitor treatment) demonstrated that this post-incubation effect is affiliated with the expression of changes associated with vaccine cell death. The cured mice acquired resistance to re-challenge with the same tumour, while the engagement of cytotoxic T lymphocytes was demonstrated by detection of high numbers of degranulating CD8+ cells in vaccinated tumours. The vaccines prepared from ex vivo PDT-treated SCCVII tumour tissue were also highly effective, implying that surgically removed tumour tissue can be directly used for PDT vaccines. This opens attractive prospects for employing PDT vaccines tailored for individual patients targeting specific antigens of the patient's tumour VSports手机版. .
Figures
References
-
- Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA (2003) Sensitive and viable identification of antigen-specific CD8+ T cells by flow cytometric assay for degranulation. J Immunol Methods 281: 65–78 - PubMed
-
- Brown SB, Brown EA, Walker I (2004) The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol 5: 497–508 - PubMed
-
- Burkett MW, Shafer-Weaver KA, Strobl S, Baseler M, Malyguine A (2005) A novel flow cytometric assay for evaluating cell-mediated cytotoxicity. J Immunother 28: 396–402 - PubMed
-
- Copier J, Dalgleish A (2006) Overview of tumour cell-based vaccines. Int Rev Immunol 25: 297–319 - PubMed
Publication types
MeSH terms
- "VSports" Actions
- V体育安卓版 - Actions
- Actions (VSports最新版本)
- "V体育平台登录" Actions
- V体育ios版 - Actions
- "V体育ios版" Actions
- Actions (VSports app下载)
- Actions (VSports)
- "V体育ios版" Actions
- "VSports app下载" Actions
Substances
- VSports注册入口 - Actions
- "V体育官网入口" Actions
LinkOut - more resources
Full Text Sources
"VSports最新版本" Other Literature Sources
Research Materials (VSports)

