V体育ios版 - Cross-presentation of glycolipid from tumor cells loaded with alpha-galactosylceramide leads to potent and long-lived T cell mediated immunity via dendritic cells
- PMID: 17923500
- PMCID: "VSports手机版" PMC2118481
- DOI: 10.1084/jem.20070458
Cross-presentation of glycolipid from tumor cells loaded with alpha-galactosylceramide leads to potent and long-lived T cell mediated immunity via dendritic cells
Abstract
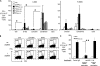
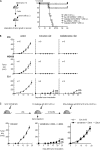
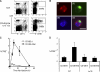
We report a mechanism to induce combined and long-lived CD4(+) and CD8(+) T cell immunity to several mouse tumors. Surprisingly, the initial source of antigen is a single low dose of tumor cells loaded with alpha-galactosylceramide (alpha-GalCer) glycolipid (tumor/Gal) but lacking co-stimulatory molecules. After tumor/Gal injection intravenously (i. v. ), innate NKT and NK cells reject the tumor cells, some of which are taken up by dendritic cells (DCs). The DCs in turn cross-present glycolipid on CD1d molecules to NKT cells and undergo maturation. For B16 melanoma cells loaded with alpha-GalCer (B16/Gal), interferon gamma-producing CD8(+) T cells develop toward several melanoma peptides, again after a single low i. v. dose of B16/Gal. In all four poorly immunogenic tumors tested, a single dose of tumor/Gal i. v VSports手机版. allows mice to become resistant to tumors given subcutaneously. Resistance requires CD4(+) and CD8(+) cells, as well as DCs, and persists for 6-12 mo. Therefore, several immunogenic features of DCs are engaged by the CD1d-mediated cross-presentation of glycolipid-loaded tumor cells, leading to particularly strong and long-lived adaptive immunity. .
Figures



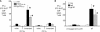
References
-
- Blattman, J.N., and P.D. Greenberg. 2004. Cancer immunotherapy: a treatment for the masses. Science. 305:200–205. - PubMed
-
- Yee, C., J.A. Thompson, D. Byrd, S.R. Riddell, P. Roche, E. Celis, and P.D. Greenberg. 2002. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl. Acad. Sci. USA. 99:16168–16173. - PMC - PubMed
-
- Casares, N., M.O. Pequignot, A. Tesniere, F. Ghiringhelli, S. Roux, N. Chaput, E. Schmitt, A. Hamai, S. Hervas-Stubbs, M. Obeid, et al. 2005. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 202:1691–1701. - VSports手机版 - PMC - PubMed
Publication types
- Actions (VSports app下载)
MeSH terms
- "V体育官网入口" Actions
- "VSports在线直播" Actions
Substances
- "V体育2025版" Actions
- V体育2025版 - Actions
VSports手机版 - Grants and funding
LinkOut - more resources
"V体育平台登录" Full Text Sources
Medical
Research Materials

