Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species
- PMID: 17914462
- PMCID: PMC2063476
- DOI: "V体育2025版" 10.1038/sj.emboj.7601867
Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species
Abstract
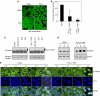
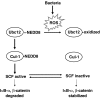
The resident prokaryotic microflora of the mammalian intestine influences diverse homeostatic functions of the gut, including regulation of cellular growth and immune responses; however, it is unknown how commensal prokaryotic organisms mechanistically influence eukaryotic signaling networks VSports手机版. We have shown that bacterial coculture with intestinal epithelial cells modulates ubiquitin-mediated degradation of important signaling intermediates, including beta-catenin and the NF-kappaB inhibitor IkappaB-alpha. Ubiquitination of these proteins as well as others is catalyzed by the SCF(betaTrCP) ubiquitin ligase, which itself requires regulated modification of the cullin-1 subunit by the ubiquitin-like protein NEDD8. Here we show that epithelia contacted by enteric commensal bacteria in vitro and in vivo rapidly generate reactive oxygen species (ROS). Bacterially induced ROS causes oxidative inactivation of the catalytic cysteine residue of Ubc12, the NEDD8-conjugating enzyme, resulting in complete but transient loss of cullin-1 neddylation and consequent effects on NF-kappaB and beta-catenin signaling. Our results demonstrate that commensal bacteria directly modulate a critical control point of the ubiquitin-proteasome system, and suggest how enteric commensal bacterial flora influences the regulatory pathways of the mammalian intestinal epithelia. .
Figures




References
-
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI (2005) Host–bacterial mutualism in the human intestine. Science 307: 1915–1920 - "VSports在线直播" PubMed
-
- Barford D (2004) The role of cysteine residues as redox-sensitive regulatory switches. Curr Opin Struct Biol 14: 679–686 - PubMed
-
- Bossis G, Melchior F (2006) Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol Cell 21: 349–357 - PubMed
-
- Cheng G, Lambeth JD (2004) NOXO1, regulation of lipid binding, localization, and activation of Nox1 by the Phox homology (PX) domain. J Biol Chem 279: 4737–4742 - PubMed
Publication types
- "VSports注册入口" Actions
"V体育官网入口" MeSH terms
- "V体育ios版" Actions
- "V体育官网" Actions
- "V体育2025版" Actions
- VSports - Actions
- VSports最新版本 - Actions
- VSports最新版本 - Actions
- Actions (V体育官网)
- Actions (VSports手机版)
- VSports在线直播 - Actions
- "V体育官网入口" Actions
- Actions (V体育ios版)
Substances
- "V体育官网" Actions
- "V体育2025版" Actions
- "V体育平台登录" Actions
- "V体育官网" Actions
- "VSports手机版" Actions
- "VSports在线直播" Actions
"VSports app下载" Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

