Biochemical basis of regulation of human copper-transporting ATPases
- PMID: 17562324
- PMCID: PMC2025638
- DOI: 10.1016/j.abb.2007.04.013
Biochemical basis of regulation of human copper-transporting ATPases
Abstract
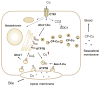
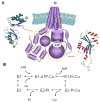
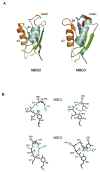
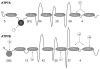
Copper is essential for cell metabolism as a cofactor of key metabolic enzymes. The biosynthetic incorporation of copper into secreted and plasma membrane-bound proteins requires activity of the copper-transporting ATPases (Cu-ATPases) ATP7A and ATP7B. The Cu-ATPases also export excess copper from the cell and thus critically contribute to the homeostatic control of copper VSports手机版. The trafficking of Cu-ATPases from the trans-Golgi network to endocytic vesicles in response to various signals allows for the balance between the biosynthetic and copper exporting functions of these transporters. Although significant progress has been made towards understanding the biochemical characteristics of human Cu-ATPase, the mechanisms that control their function and intracellular localization remain poorly understood. In this review, we summarize current information on structural features and functional properties of ATP7A and ATP7B. We also describe sequence motifs unique for each Cu-ATPase and speculate about their role in regulating ATP7A and ATP7B activity and trafficking. .
Figures

References
-
- Petris MJ, Strausak D, Mercer JF. Hum Mol Genet. 2000;9(19):2845–2851. - "V体育官网" PubMed
-
- Steveson TC, Ciccotosto GD, Ma XM, Mueller GP, Mains RE, Eipper BA. Endocrinology. 2003;144(1):188–200. - PubMed
-
- Tchaparian EH, Uriu-Adams JY, Keen CL, Mitchell AE, Rucker RB. Arch Biochem Biophys. 2000;379(1):71–77. - PubMed
-
- Qin Z, Itoh S, Jeney V, Ushio-Fukai M, Fukai T. Faseb J. 2006;20(2):334–336. - VSports手机版 - PubMed
-
- Terada K, Nakako T, Yang XL, Iida M, Aiba N, Minamiya Y, Nakai M, Sakaki T, Miura N, Sugiyama T. J Biol Chem. 1998;273(3):1815–1820. - PubMed
Publication types (V体育ios版)
- Actions (VSports在线直播)
MeSH terms
- "V体育平台登录" Actions
- VSports注册入口 - Actions
- "VSports在线直播" Actions
- "V体育平台登录" Actions
- "V体育ios版" Actions
- Actions (VSports最新版本)
- VSports在线直播 - Actions
Substances
- "VSports" Actions
- Actions (VSports最新版本)
- Actions (VSports手机版)
- VSports手机版 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources (VSports最新版本)
Miscellaneous

