"VSports最新版本" CIDE-A is expressed in liver of old mice and in type 2 diabetic mouse liver exhibiting steatosis
- PMID: 17472743
- PMCID: PMC1868770
- DOI: 10.1186/1476-5926-6-4
"VSports手机版" CIDE-A is expressed in liver of old mice and in type 2 diabetic mouse liver exhibiting steatosis
Abstract
Background: Increased levels of circulating fatty acids caused by insulin resistance and increased adipocyte lipolysis can accumulate within the liver resulting in steatosis. This steatosis sensitizes the liver to inflammation and further injury which can lead to liver dysfunction. We performed microarray analysis on normal mouse liver tissue at different ages and type 2 diabetic liver exhibiting steatosis to identify differentially expressed genes involved in lipid accumulation and liver dysfunction. VSports手机版.
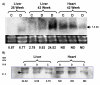
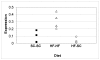
Results: Microarray analysis identified CIDE-A as the most differentially expressed gene as a function of age. Mice fed a high fat diet developed hyperinsulinemia, hyperglycemia and liver steatosis, all features of the human metabolic syndrome. Increased CIDE-A expression was observed in type 2 diabetic liver and the elevated CIDE-A expression could be reversed by weight loss and normalization of plasma insulin. Also, CIDE-A expression was found to be correlated with hepatic lipid accumulation V体育安卓版. .
Conclusion: The corresponding increase in CIDE-A expression with hyperinsulinemia and liver steatosis suggests a novel pathway for lipid accumulation in the liver. V体育ios版.
Figures





References
-
- Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. - V体育2025版 - PubMed
-
- Natori S, Rust C, Stadheim LM, Srinivasan A, Burgart LJ, Gores GJ. Hepatocyte apoptosis is a pathologic feature of human alcoholic hepatitis. J Hepatol. 2001;34:248–253. doi: 10.1016/S0168-8278(00)00089-1. - DOI (V体育ios版) - PubMed
"V体育2025版" Grants and funding
LinkOut - more resources
Full Text Sources (VSports最新版本)
