The oxidoreductase DsbA plays a key role in the ability of the Crohn's disease-associated adherent-invasive Escherichia coli strain LF82 to resist macrophage killing
- PMID: 17449627
- PMCID: PMC1913465
- DOI: 10.1128/JB.00233-07
The oxidoreductase DsbA plays a key role in the ability of the Crohn's disease-associated adherent-invasive Escherichia coli strain LF82 to resist macrophage killing
Abstract
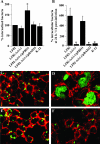
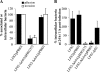
Adherent-invasive Escherichia coli (AIEC) isolated from Crohn's disease patients is able to adhere to and invade intestinal epithelial cells and to replicate in mature phagolysosomes within macrophages VSports手机版. Here, we show that the dsbA gene, encoding a periplasmic oxidoreductase, was required for AIEC strain LF82 to adhere to intestinal epithelial cells and to survive within macrophages. The LF82-DeltadsbA mutant did not express flagella and, probably as a consequence of this, did not express type 1 pili. The role of DsbA in adhesion is restricted to the loss of flagella and type 1 pili, as forced contact between bacteria and cells and induced expression of type 1 pili restored the wild-type phenotype. In contrast, the dsbA gene is essential for AIEC LF82 bacteria to survive within macrophages, irrespective of the loss of flagella and type 1 pilus expression, and the survival ability of LF82-DeltadsbA was as low as that of the nonpathogenic E. coli K-12, which was efficiently killed by macrophages. We also provide evidence that the dsbA gene is needed for LF82 bacteria to grow and survive in an acidic and nutrient-poor medium that partly mimics the harsh environment of the phagocytic vacuole. In addition, under such stress conditions dsbA transcription is highly up-regulated. Finally, the CpxRA signaling pathway does not play a role in regulation of dsbA expression in AIEC LF82 bacteria under conditions similar to those of mature phagolysosomes. .
V体育平台登录 - Figures

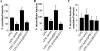


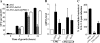
References
-
- Bardwell, J. C., K. McGovern, and J. Beckwith. 1991. Identification of a protein required for disulfide bond formation in vivo. Cell 67:581-589. - PubMed
-
- Barnich, N., J. Boudeau, L. Claret, and A. Darfeuille-Michaud. 2003. Regulatory and functional co-operation of flagella and type 1 pili in adhesive and invasive abilities of AIEC strain LF82 isolated from a patient with Crohn's disease. Mol. Microbiol. 48:781-794. - PubMed
-
- Barnich, N., M. A. Bringer, L. Claret, and A. Darfeuille-Michaud. 2004. Involvement of lipoprotein NlpI in the virulence of adherent invasive Escherichia coli strain LF82 isolated from a patient with Crohn's disease. Infect. Immun. 72:2484-2493. - "V体育2025版" PMC - PubMed
-
- Belin, P., and P. L. Boquet. 1994. The Escherichia coli dsbA gene is partly transcribed from the promoter of a weakly expressed upstream gene. Microbiology 140:3337-3348. - PubMed
Publication types
- "VSports注册入口" Actions
MeSH terms
- Actions (VSports最新版本)
- VSports最新版本 - Actions
- VSports app下载 - Actions
- "V体育官网" Actions
- Actions (VSports最新版本)
- "VSports app下载" Actions
- "V体育官网" Actions
- VSports - Actions
- "VSports" Actions
- "VSports" Actions
- V体育安卓版 - Actions
- "VSports最新版本" Actions
- "V体育平台登录" Actions
Substances
LinkOut - more resources
"V体育官网" Full Text Sources
Medical

