Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression
- PMID: 17415413
- PMCID: PMC1838926
- DOI: "VSports" 10.1172/JCI30740
Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression
Erratum in
-
"V体育安卓版" Inhibition of TGF-β with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression.J Clin Invest. 2017 Mar 1;127(3):1116. doi: 10.1172/JCI93333. Epub 2017 Mar 1. J Clin Invest. 2017. PMID: 28248204 Free PMC article. No abstract available.
Abstract
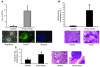
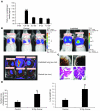
We investigated whether TGF-beta induced by anticancer therapies accelerates tumor progression. Using the MMTV/PyVmT transgenic model of metastatic breast cancer, we show that administration of ionizing radiation or doxorubicin caused increased circulating levels of TGF-beta1 as well as increased circulating tumor cells and lung metastases. These effects were abrogated by administration of a neutralizing pan-TGF-beta antibody VSports手机版. Circulating polyomavirus middle T antigen-expressing tumor cells did not grow ex vivo in the presence of the TGF-beta antibody, suggesting autocrine TGF-beta is a survival signal in these cells. Radiation failed to enhance lung metastases in mice bearing tumors that lack the type II TGF-beta receptor, suggesting that the increase in metastases was due, at least in part, to a direct effect of TGF-beta on the cancer cells. These data implicate TGF-beta induced by anticancer therapy as a pro-metastatic signal in tumor cells and provide a rationale for the simultaneous use of these therapies in combination with TGF-beta inhibitors. .
"VSports最新版本" Figures
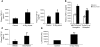
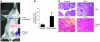

References
-
- Massague J., Seoane J., Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. - PubMed
-
- Siegel P.M., Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat. Rev. Cancer. 2003;3:807–820. - PubMed
-
- Bierie B., Moses H.L. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat. Rev. Cancer. 2006;6:506–520. - PubMed
-
- Dumont N., Arteaga C.L. Targeting the TGF beta signaling network in human neoplasia. Cancer Cell. 2003;3:531–536. - "VSports最新版本" PubMed
-
- Wakefield L.M., Roberts A.B. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr. Opin. Genet. Dev. 2002;12:22–29. - "V体育官网" PubMed
Publication types (V体育2025版)
- "V体育2025版" Actions
V体育2025版 - MeSH terms
- VSports在线直播 - Actions
- "VSports手机版" Actions
- VSports - Actions
- VSports在线直播 - Actions
- Actions (VSports app下载)
- Actions (V体育平台登录)
- Actions (VSports注册入口)
- V体育2025版 - Actions
- V体育官网入口 - Actions
- "V体育官网入口" Actions
- Actions (V体育2025版)
- V体育官网 - Actions
- "VSports app下载" Actions
- "VSports最新版本" Actions
- Actions (VSports注册入口)
- V体育官网 - Actions
Substances
- "V体育ios版" Actions
- "VSports最新版本" Actions
- "V体育安卓版" Actions
"V体育官网" Grants and funding
- R01 CA062212/CA/NCI NIH HHS/United States
- R01 CA 62212/CA/NCI NIH HHS/United States (VSports在线直播)
- R01 CA 102162/CA/NCI NIH HHS/United States
- P50 CA098131/CA/NCI NIH HHS/United States
- R01 CA102162/CA/NCI NIH HHS/United States
- VSports手机版 - P30 CA 68485/CA/NCI NIH HHS/United States
- "VSports注册入口" P30 CA068485/CA/NCI NIH HHS/United States
- R01 CA 38079/CA/NCI NIH HHS/United States
- P50 CA 98131/CA/NCI NIH HHS/United States
- R01 CA 85492/CA/NCI NIH HHS/United States
- R01 CA038079/CA/NCI NIH HHS/United States
- R01 CA085492/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical (VSports app下载)
Molecular Biology Databases

