Beta-catenin regulates positive selection of thymocytes but not lineage commitment
- PMID: 17404285
- PMCID: "V体育官网入口" PMC2274003
- DOI: 10.4049/jimmunol.178.8.5028
Beta-catenin regulates positive selection of thymocytes but not lineage commitment
Abstract
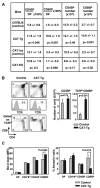
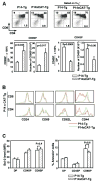
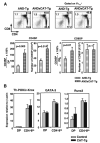
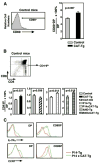
Positive selection and lineage commitment to the cytolytic or helper lineage of T cells result in coordinated expression of MHC class I-restricted TCR and CD8 coreceptor or MHC class II-restricted TCR and CD4 molecule. Positive selection signals also regulate the survival and generation of requisite numbers of cytolytic or Th cells. beta-Catenin is the major transcriptional cofactor of T cell factor and plays a role in thymocyte development VSports手机版. In this study, using mice expressing stabilized beta-catenin and mice with T cell-specific deletion of beta-catenin, we show that beta-catenin regulates positive selection, but not lineage commitment of thymocytes. Furthermore, beta-catenin expression accelerates the timing of mature CD8 thymocyte generation such that CD4 and CD8 single-positive thymocytes mature with the same kinetics during development. .
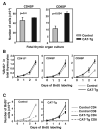
Figures

"VSports最新版本" References
-
- Jameson SC, Hogquist KA, Bevan MJ. Positive selection of thymocytes. Annu Rev Immunol. 1995;13:93–126. - PubMed
-
- Fowlkes BJ, Schweighoffer E. Positive selection of T cells. Curr Opin Immunol. 1995;7:188–195. - PubMed
-
- Bosselut R. CD4/CD8-lineage differentiation in the thymus: from nuclear effectors to membrane signals. Nat Rev Immunol. 2004;4:529–540. - V体育平台登录 - PubMed
-
- Germain RN. T-cell development and the CD4-CD8 lineage decision. Nat Rev Immunol. 2002;2:309–322. - PubMed
-
- Singer A, Bosselut R. CD4/CD8 coreceptors in thymocyte development, selection, and lineage commitment: analysis of the CD4/CD8 lineage decision. Adv Immunol. 2004;83:91–131. - "VSports手机版" PubMed
"V体育平台登录" Publication types
MeSH terms
- "VSports" Actions
- Actions (VSports最新版本)
- Actions (VSports注册入口)
- "VSports注册入口" Actions
Substances (VSports在线直播)
- V体育官网 - Actions
- VSports最新版本 - Actions
Grants and funding (VSports)
LinkOut - more resources
V体育2025版 - Full Text Sources
Molecular Biology Databases (V体育官网入口)
V体育官网 - Research Materials

