Superoxide flux in endothelial cells via the chloride channel-3 mediates intracellular signaling
- PMID: 17360969
- PMCID: V体育2025版 - PMC1877121
- DOI: 10.1091/mbc.e06-09-0830
Superoxide flux in endothelial cells via the chloride channel-3 mediates intracellular signaling (V体育ios版)
VSports在线直播 - Abstract
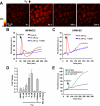
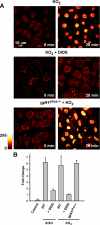
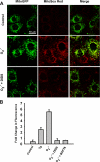
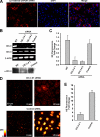
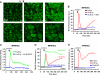
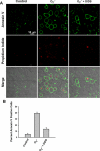
Reactive oxygen species (ROS) have been implicated in both cell signaling and pathology. A major source of ROS in endothelial cells is NADPH oxidase, which generates superoxide (O(2)(. -)) on the extracellular side of the plasma membrane but can result in intracellular signaling. To study possible transmembrane flux of O(2)(. -), pulmonary microvascular endothelial cells were preloaded with the O(2)(. -)-sensitive fluorophore hydroethidine (HE). Application of an extracellular bolus of O(2)(. -) resulted in rapid and concentration-dependent transient HE oxidation that was followed by a progressive and nonreversible increase in nuclear HE fluorescence. These fluorescence changes were inhibited by superoxide dismutase (SOD), the anion channel blocker DIDS, and selective silencing of the chloride channel-3 (ClC-3) by treatment with siRNA VSports手机版. Extracellular O(2)(. -) triggered Ca(2+) release in turn triggered mitochondrial membrane potential alterations that were followed by mitochondrial O(2)(. -) production and cellular apoptosis. These "signaling" effects of O(2)(. -) were prevented by DIDS treatment, by depletion of intracellular Ca(2+) stores with thapsigargin and by chelation of intracellular Ca(2+). This study demonstrates that O(2)(. -) flux across the endothelial cell plasma membrane occurs through ClC-3 channels and induces intracellular Ca(2+) release, which activates mitochondrial O(2)(. -) generation. .
Figures
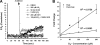

References
-
- Aon M. A., Cortassa S., Marban E., O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J. Biol. Chem. 2003;278:44735–44744. - PubMed (VSports注册入口)
-
- Babior B. M. NADPH oxidase: an update. Blood. 1999;93:1464–1476. - PubMed
-
- Becker L. B., vanden Hoek T. L., Shao Z. H., Li C. Q., Schumacker P. T. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am. J. Physiol. 1999;277:H2240–H2246. - PubMed
-
- Devadas S., Zaritskaya L., Rhee S. G., Oberley L., Williams M. S. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J. Exp. Med. 2002;195:59–70. - "V体育官网" PMC - PubMed
-
- Fink B., Laude K., McCann L., Doughan A., Harrison D. G., Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am. J. Physiol. Cell Physiol. 2004;287:C895–C902. - "V体育2025版" PubMed
Publication types
- VSports最新版本 - Actions
MeSH terms
- VSports - Actions
- "V体育安卓版" Actions
- V体育官网入口 - Actions
- V体育平台登录 - Actions
- Actions (VSports注册入口)
- VSports手机版 - Actions
- V体育官网 - Actions
- "V体育官网" Actions
Substances
- "VSports最新版本" Actions
- "VSports注册入口" Actions
- Actions (V体育安卓版)
- Actions (VSports app下载)
- "VSports" Actions
- "V体育安卓版" Actions
- V体育官网 - Actions
- "VSports" Actions
Grants and funding
LinkOut - more resources (VSports在线直播)
Full Text Sources
Other Literature Sources
Miscellaneous

