Competition for antigen determines the stability of T cell-dendritic cell interactions during clonal expansion
- PMID: 17360562
- PMCID: PMC1838639
- DOI: 10.1073/pnas.0610019104
VSports注册入口 - Competition for antigen determines the stability of T cell-dendritic cell interactions during clonal expansion
Abstract
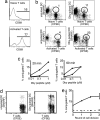
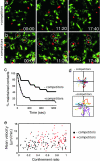
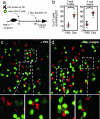
The regulation of T cell-dendritic cell (DC) contacts during clonal expansion is poorly defined. Although optimal CD4 T cell responses require prolonged exposure to antigen (Ag), it is believed that stable T cell-DC interactions occur only during the first day of the activation process. Here we show that recently activated CD4 T cells are in fact fully competent for establishing contact with Ag-bearing DC. Using two-photon imaging, we found that whereas prolonged interactions between activated T cells and Ag-bearing DCs were infrequent at high T cell precursor frequency, they were readily observed for a period of at least 2 days when lower numbers of T cells were used. We provide evidence that, when present in high numbers, Ag-specific T cells still gained access to the DC surface but were competing for the limited number of sites on DCs with sufficient peptide-MHC complexes for the establishment of a long-lived interaction. Consistent with these findings, we showed that restoration of peptide-MHC level on DCs at late time points was sufficient to recover interactions between activated T cells and DCs. Thus, the period during which CD4 T cells continue to establish stable interactions with DCs is longer than previously thought, and its duration is dictated by both Ag levels and T cell numbers, providing a feedback mechanism for the termination of CD4 T cell responses. VSports手机版.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Breart B, Bousso P. Curr Opin Immunol. 2006;18:483–490. - PubMed
-
- Mempel TR, Henrickson SE, Von Andrian UH. Nature. 2004;427:154–159. - PubMed
-
- Hugues S, Fetler L, Bonifaz L, Helft J, Amblard F, Amigorena S. Nat Immunol. 2004;5:1235–1242. - PubMed
-
- Zinselmeyer BH, Dempster J, Gurney AM, Wokosin D, Miller M, Ho H, Millington OR, Smith KM, Rush CM, Parker I, et al. J Exp Med. 2005;201:1815–1823. - V体育2025版 - PMC - PubMed
Publication types
- Actions (VSports)
MeSH terms (VSports注册入口)
- "VSports app下载" Actions
- "V体育官网入口" Actions
- "V体育2025版" Actions
- Actions (VSports最新版本)
- "VSports手机版" Actions
- "V体育平台登录" Actions
- "V体育2025版" Actions
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
VSports在线直播 - Research Materials

