Ancestry and pharmacogenetics of antileukemic drug toxicity
- PMID: 17264302
- PMCID: PMC1885506
- DOI: 10.1182/blood-2006-10-054528
Ancestry and pharmacogenetics of antileukemic drug toxicity
Abstract
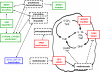
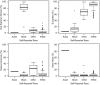
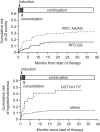
Treatment-related toxicity in acute lymphoblastic leukemia (ALL) can not only be life threatening but may also affect relapse risk. In 240 patients, we determined whether toxicities were related to 16 polymorphisms in genes linked to the pharmacodynamics of ALL chemotherapy, adjusting for age, race (self-reported or via ancestry-informative markers), sex, and disease risk group (lower- vs higher-risk therapy). Toxicities (gastrointestinal, infectious, hepatic, and neurologic) were assessed in each treatment phase. During the induction phase, when drugs subject to the steroid/cytochrome P4503A pathway predominated, genotypes in that pathway were important: vitamin D receptor (odds ratio [OR], 6. 85 [95% confidence interval [CI], 1. 73-27. 0]) and cytochrome P4503A5 (OR, 4. 61 [95% CI, 1. 11-19. 2]) polymorphisms were related to gastrointestinal toxicity and infection, respectively. During the consolidation phase, when antifolates predominated, the reduced folate carrier polymorphism predicted gastrointestinal toxicity (OR, 10. 4 [95% CI, 1. 35-80. 4]) as it also did during continuation (OR, 2. 01 [95% CI, 1 VSports手机版. 06-4. 11]). In all 3 treatment phases, a glucuronosyltransferase polymorphism predicted hyperbilirubinemia (P = . 017, P < . 001, and P < . 001) and methotrexate clearance (P = . 028), which was also independently associated with hyperbilirubinemia (P = . 026). The genotype-phenotype associations were similar whether analyses were adjusted by self-reported race or ancestry-informative genetic markers. Germ-line polymorphisms are significant determinants of toxicity of antileukemic therapy. .
Figures

References
-
- Nachman JB, Sather HN, Sensel MG, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338:1663–1671. - PubMed
-
- Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004;350:1535–1548. - PubMed
-
- Schrappe M, Camitta B, Pui CH, et al. Long-term results of large prospective trials in childhood acute lymphoblastic leukemia. Leukemia. 2000;14:2193–2194. - PubMed
-
- Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97:1211–1218. - PubMed
-
- Rivera GK, Evans WE, Kalwinsky DK, et al. Unexpectedly severe toxicity from intensive early treatment of childhood lymphoblastic leukemia. J Clin Oncol. 1985;3:201–206. - PubMed
Publication types
- V体育ios版 - Actions
MeSH terms
- V体育平台登录 - Actions
- V体育官网入口 - Actions
- VSports在线直播 - Actions
- VSports手机版 - Actions
- "V体育2025版" Actions
- "V体育官网" Actions
- V体育官网入口 - Actions
- VSports最新版本 - Actions
- Actions (VSports app下载)
- V体育官网 - Actions
Substances
"V体育安卓版" Grants and funding
VSports在线直播 - LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

