Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host
- PMID: 17244679
- PMCID: PMC1885503
- DOI: 10.1182/blood-2006-09-046201
Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host (VSports)
Abstract
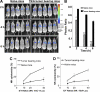
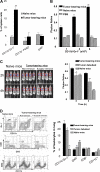
Tumor growth promotes the expansion of myeloid suppressor cells. An inverse correlation between natural killer (NK) cell activation and myeloid suppressor cell (MSC) expansion in tumor-bearing patients and mice prompted us to investigate the role of MSCs in controlling NK antitumor cytotocixity. After adoptive transfer to naive recipients, CD11b(+)Gr-1(+) MSCs freshly isolated from spleens of tumor-bearing mice but not naive mice were able to inhibit NK cell cytotoxicity. An in vivo imaging analysis indicates that the removal of tumors resulted in a significant increased ability (P < VSports手机版. 05) in NK cell cytotoxicity to eliminate injected YAC-1 cells from the lungs. Fluorescence-activated cell sorter (FACS) analysis of the composition of lung leukocytes further indicates that the removal of tumors also leads to the reduction of MSCs accumulated in the lung. These data suggest that MSCs suppress NK cell cytotoxicity. The inhibition of NK cell cytotoxicity is cell-cell contact dependent. Inhibition of perforin but not granzyme B production was responsible for MSC-mediated inhibition of NK cytotoxicity. Western blot analyses further suggests that MSCs suppress IL-2-mediated NK cell cytotoxicity by affecting the activity of Stat5. .
VSports app下载 - Figures
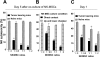


References (VSports app下载)
-
- Valenti R, Huber V, Filipazzi P, et al. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 2006;66:9290–9298. - PubMed (V体育ios版)
-
- Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. - PubMed
Publication types
- "VSports在线直播" Actions
- "VSports在线直播" Actions
MeSH terms
- "V体育ios版" Actions
- Actions (V体育平台登录)
- V体育平台登录 - Actions
- V体育官网入口 - Actions
- "V体育平台登录" Actions
- VSports最新版本 - Actions
Substances
- "VSports在线直播" Actions
- "VSports手机版" Actions
- "V体育安卓版" Actions
- Actions (VSports注册入口)
- "VSports最新版本" Actions
"VSports手机版" Grants and funding
"VSports手机版" LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
V体育官网 - Miscellaneous

