Mechanisms underlying the resistance to diet-induced obesity in germ-free mice
- PMID: 17210919
- PMCID: PMC1764762 (VSports)
- DOI: VSports在线直播 - 10.1073/pnas.0605374104
Mechanisms underlying the resistance to diet-induced obesity in germ-free mice
Abstract
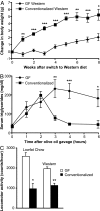
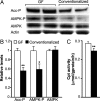
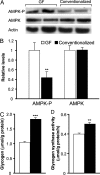
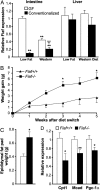
The trillions of microbes that colonize our adult intestines function collectively as a metabolic organ that communicates with, and complements, our own human metabolic apparatus. Given the worldwide epidemic in obesity, there is interest in how interactions between human and microbial metabolomes may affect our energy balance. Here we report that, in contrast to mice with a gut microbiota, germ-free (GF) animals are protected against the obesity that develops after consuming a Western-style, high-fat, sugar-rich diet. Their persistently lean phenotype is associated with increased skeletal muscle and liver levels of phosphorylated AMP-activated protein kinase (AMPK) and its downstream targets involved in fatty acid oxidation (acetylCoA carboxylase; carnitine-palmitoyltransferase). Moreover, GF knockout mice lacking fasting-induced adipose factor (Fiaf), a circulating lipoprotein lipase inhibitor whose expression is normally selectively suppressed in the gut epithelium by the microbiota, are not protected from diet-induced obesity. Although GF Fiaf-/- animals exhibit similar levels of phosphorylated AMPK as their wild-type littermates in liver and gastrocnemius muscle, they have reduced expression of genes encoding the peroxisomal proliferator-activated receptor coactivator (Pgc-1alpha) and enzymes involved in fatty acid oxidation. Thus, GF animals are protected from diet-induced obesity by two complementary but independent mechanisms that result in increased fatty acid metabolism: (i) elevated levels of Fiaf, which induces Pgc-1alpha; and (ii) increased AMPK activity. Together, these findings support the notion that the gut microbiota can influence both sides of the energy balance equation, and underscore the importance of considering our metabolome in a supraorganismal context. VSports手机版.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References (V体育平台登录)
-
- Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Science. 2005;307:1915–1920. - PubMed
-
- Wolin M. Science. 1981;213:1463–1468. - PubMed
-
- Merkel M, Eckel RH, Goldberg IJ. J Lipid Res. 2002;43:1997–2006. - VSports最新版本 - PubMed
-
- Preiss-Landl K, Zimmermann R, Hammerle G, Zechner R. Curr Opin Lipidol. 2002;13:471–481. - PubMed
Publication types
MeSH terms
- Actions (V体育平台登录)
- Actions (VSports在线直播)
- "VSports app下载" Actions
- Actions (VSports最新版本)
- "V体育官网" Actions
- Actions (VSports注册入口)
- Actions (VSports注册入口)
- "V体育ios版" Actions
V体育平台登录 - Substances
- Actions (VSports app下载)
- "VSports最新版本" Actions
- VSports - Actions
- "VSports在线直播" Actions
- V体育安卓版 - Actions
- "VSports注册入口" Actions
- VSports最新版本 - Actions
- "V体育平台登录" Actions
Grants and funding (VSports注册入口)
LinkOut - more resources (VSports在线直播)
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

