Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes (VSports手机版)
- PMID: 17182262
- PMCID: PMC1805710 (V体育官网入口)
- DOI: V体育官网 - 10.1016/j.immuni.2006.10.018
Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes
Abstract
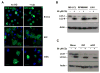
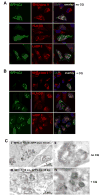
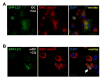
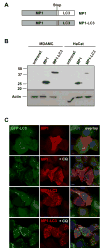
Major histocompatibility complex (MHC) class II molecules present products of lysosomal proteolysis to CD4(+) T cells. Although extracellular antigen uptake is considered to be the main source of MHC class II ligands, a few intracellular antigens have been described to gain access to MHC class II loading after macroautophagy. However, the general relevance and efficacy of this pathway is unknown VSports手机版. Here we demonstrated constitutive autophagosome formation in MHC class II-positive cells, including dendritic, B, and epithelial cells. The autophagosomes continuously fuse with multivesicular MHC class II-loading compartments. This pathway was of functional relevance, because targeting of the influenza matrix protein 1 to autophagosomes via fusion to the autophagosome-associated protein Atg8/LC3 led to strongly enhanced MHC class II presentation to CD4(+) T cell clones. We suggest that macroautophagy constitutively and efficiently delivers cytosolic proteins for MHC class II presentation and can be harnessed for improved helper T cell stimulation. .
Figures




V体育ios版 - Comment in
-
Autophagy in MHC class II presentation: sampling from within.Immunity. 2007 Jan;26(1):1-3. doi: 10.1016/j.immuni.2007.01.005. Immunity. 2007. PMID: 17241953 Review.
"V体育ios版" References
-
- Appay V, Zaunders JJ, Papagno L, Sutton J, Jaramillo A, Waters A, Easterbrook P, Grey P, Smith D, McMichael AJ, et al. Characterization of CD4+ CTLs ex vivo. J Immunol. 2002;168:5954–5958. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Berg TO, Fengsrud M, Stromhaug PE, Berg T, Seglen PO. Isolation and characterization of rat liver amphisomes. Evidence for fusion of autophagosomes with both early and late endosomes. J Biol Chem. 1998;273:21883–21892. - PubMed
-
- Bevan MJ. Helping the CD8+ T-cell response. Nat Rev Immunol. 2004;4:595–602. - PubMed
-
- Brazil MI, Weiss S, Stockinger B. Excessive degradation of intracellular protein in macrophages prevents presentation in the context of major histocompatibility complex class II molecules. Eur J Immunol. 1997;27:1506–1514. - PubMed (VSports手机版)
Publication types
- "VSports在线直播" Actions
MeSH terms
- Actions (VSports手机版)
- "VSports手机版" Actions
- Actions (VSports注册入口)
- Actions (VSports最新版本)
- Actions (V体育安卓版)
- Actions (VSports在线直播)
- "VSports" Actions
- V体育平台登录 - Actions
- "VSports最新版本" Actions
- "V体育官网" Actions
Substances
- "V体育ios版" Actions
Grants and funding
LinkOut - more resources (V体育官网入口)
Full Text Sources
Other Literature Sources
V体育安卓版 - Molecular Biology Databases
"VSports在线直播" Research Materials

