Palmitoylation of CD95 facilitates formation of SDS-stable receptor aggregates that initiate apoptosis signaling
- PMID: 17159907
- PMCID: PMC1782382
- DOI: VSports在线直播 - 10.1038/sj.emboj.7601460
Palmitoylation of CD95 facilitates formation of SDS-stable receptor aggregates that initiate apoptosis signaling
Abstract
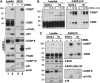
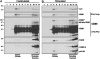
Apoptosis signaling through CD95 (Fas/APO-1) involves aggregation and clustering of the receptor followed by its actin-dependent internalization. Internalization is required for efficient formation of the death-inducing signaling complex (DISC) with maximal recruitment of FADD, caspase-8/10 and c-FLIP occurring when the receptor has reached an endosomal compartment. The first detectable event during CD95 signaling is the formation of SDS-stable aggregates likely reflecting intense oligomerization of the receptor. We now demonstrate that these SDS-stable forms of CD95 correspond to very high molecular weight DISC complexes (hiDISC) and are the sites of caspase-8 activation. hiDISCs are found both inside and outside of detergent-resistant membranes. The formation of SDS-stable CD95 aggregates involves palmitoylation of the membrane proximal cysteine 199 in CD95. Cysteine 199 mutants no longer form SDS-stable aggregates, and inhibition of palmitoylation reduces internalization of CD95 and activation of caspase-8 VSports手机版. Our data demonstrate that SDS-stable forms of CD95 are the sites of apoptosis initiation and represent an important early step in apoptosis signaling through CD95 before activation of caspases. .
Figures (V体育平台登录)
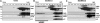
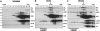



V体育平台登录 - References
-
- Algeciras-Schimnich A, Peter ME (2003) Actin dependent CD95 internalization is specific for Type I cells. FEBS Lett 546: 185–188 - PubMed
-
- Algeciras-Schimnich A, Shen L, Barnhart BC, Murmann AE, Burkhardt JK, Peter ME (2002) Molecular ordering of the initial signaling events of CD95. Mol Cell Biol 22: 207–220 - VSports在线直播 - PMC - PubMed
-
- Barnhart BC, Alappat EC, Peter ME (2003) The CD95 type I/type II model. Semin Immunol 15: 185–193 - PubMed (VSports)
-
- Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, Salvesen GS (2003) A unified model for apical caspase activation. Mol Cell 11: 529–541 - PubMed
Publication types
- Actions (VSports最新版本)
- "VSports" Actions
MeSH terms
- "V体育平台登录" Actions
- Actions (V体育2025版)
- "VSports手机版" Actions
- "V体育2025版" Actions
- "V体育官网入口" Actions
- Actions (V体育平台登录)
- V体育ios版 - Actions
"VSports注册入口" Substances
- VSports注册入口 - Actions
- "VSports注册入口" Actions
Grants and funding
VSports最新版本 - LinkOut - more resources
Full Text Sources
"V体育平台登录" Other Literature Sources
VSports最新版本 - Molecular Biology Databases
Research Materials
Miscellaneous

