A critical role for the loop region of the basic helix-loop-helix/leucine zipper protein Mlx in DNA binding and glucose-regulated transcription
- PMID: 17148476
- PMCID: PMC1761440
- DOI: "V体育安卓版" 10.1093/nar/gkl987
A critical role for the loop region of the basic helix-loop-helix/leucine zipper protein Mlx in DNA binding and glucose-regulated transcription
Abstract
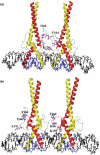
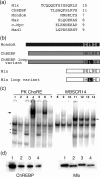
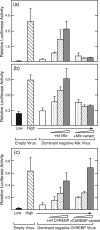
The carbohydrate response element (ChoRE) is a cis-acting sequence found in the promoters of genes induced transcriptionally by glucose. The ChoRE is composed of two E box-like motifs that are separated by 5 bp and is recognized by two basic helix-loop-helix/leucine zipper (bHLH/LZ) proteins, ChREBP and Mlx, which heterodimerize to bind DNA. In this study, we demonstrate that two ChREBP/Mlx heterodimers interact to stabilize binding to the tandem E box-like motifs in the ChoRE VSports手机版. Based on a model structure that we generated of ChREBP/Mlx bound to the ChoRE, we hypothesized that intermolecular interactions between residues within the Mlx loop regions of adjacent heterodimers are responsible for stabilizing the complex. We tested this hypothesis by preparing Mlx variants in which the loop region was replaced with that of another family member or mutated at several key residues. These Mlx variants retained their ability to bind to a single perfect E-box motif as a heterodimer with ChREBP, but no longer bound to the ChoRE nor supported glucose responsive activity. In summary, our results support a model in which the loop regions of Mlx play an important functional role in mediating the coordinate binding of ChREBP/Mlx heterodimers to the ChoRE. .
Figures

"VSports注册入口" References
-
- Towle H.C., Kaytor E.N., Shih H.-M. Regulation of the expression of lipogenic enzyme genes by carbohydrate. Annu. Rev. Nutr. 1997;17:405–433. - PubMed
-
- Girard J., Ferre P., Foufelle F. Mechanisms by which carbohydrates regulate expression of genes for glycolytic and lipogenic enzymes. Annu. Rev. Nutr. 1997;17:325–352. - PubMed
-
- Vaulont S., Vasseur-Cognet M., Kahn A. Glucose regulation of gene transcription. J. Biol. Chem. 2000;275:31555–31558. - PubMed
-
- Towle H.C. Glucose as a regulator of eukaryotic gene transcription. Trends Endocrinol. Metab. 2005;16:489–494. - "V体育平台登录" PubMed
-
- O'Callaghan B.L., Koo S.-H., Wu Y., Freake H.C., Towle H.C. Glucose regulation of the acetyl-CoA carboxylase promoter PI in rat hepatocytes. J. Biol. Chem. 2001;276:16033–16039. - PubMed (V体育ios版)
Publication types
MeSH terms
- Actions (V体育平台登录)
- Actions (VSports app下载)
- Actions (VSports app下载)
- "V体育官网" Actions
- "V体育官网" Actions
- "VSports最新版本" Actions
- Actions (V体育官网入口)
- "V体育2025版" Actions
- "VSports注册入口" Actions
- "V体育平台登录" Actions
Substances
- "VSports在线直播" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

