Anionic lipids enriched at the ExPortal of Streptococcus pyogenes
- PMID: 17142392
- PMCID: PMC1797331
- DOI: 10.1128/JB.01549-06 (V体育平台登录)
Anionic lipids enriched at the ExPortal of Streptococcus pyogenes
Abstract
The ExPortal of Streptococcus pyogenes is a membrane microdomain dedicated to the secretion and folding of proteins. We investigated the lipid composition of the ExPortal by examining the distribution of anionic membrane phospholipids VSports手机版. Staining with 10-N-nonyl-acridine orange revealed a single microdomain enriched with an anionic phospholipid whose staining characteristics and behavior in a cardiolipin-deficient mutant were characteristic of phosphatidylglycerol. Furthermore, the location of the microdomain corresponded to the site of active protein secretion at the ExPortal. These results indicate that the ExPortal is an asymmetric lipid microdomain, whose enriched content of anionic phospholipids may play an important role in ExPortal organization and protein trafficking. .
Figures
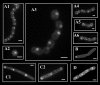

References
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
-
- Cajal, Y., J. Ghanta, K. Easwaran, A. Surolia, and M. K. Jain. 1996. Specificity for the exchange of phospholipids through polymyxin B mediated intermembrane molecular contacts. Biochemistry 35:5684-5695. - PubMed
-
- Campo, N., H. Tjalsma, G. Buist, D. Stepniak, M. Meijer, M. Veenhuis, M. Westermann, J. P. Muller, S. Bron, J. Kok, O. P. Kuipers, and J. D. H. Jongbloed. 2004. Subcellular sites for bacterial protein export. Mol. Microbiol. 53:1583-1599. - "VSports最新版本" PubMed
-
- Caparon, M. 2000. Gram-positive pathogens. ASM Press, Washington, DC.
VSports app下载 - Publication types
MeSH terms
- "VSports注册入口" Actions
- "V体育官网入口" Actions
- "VSports" Actions
- "V体育2025版" Actions
VSports在线直播 - Substances
- Actions (V体育官网)
- Actions (V体育官网入口)
- VSports手机版 - Actions
"VSports最新版本" Grants and funding
LinkOut - more resources
Full Text Sources (VSports app下载)
Other Literature Sources

