"V体育官网入口" An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium
- PMID: 17116732
- PMCID: PMC2118156
- DOI: VSports - 10.1084/jem.20051759
"V体育官网" An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium
Abstract
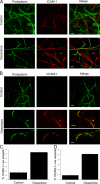
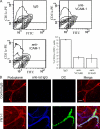
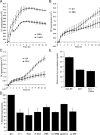
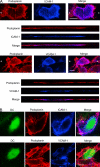
The exit of antigen-presenting cells and lymphocytes from inflamed skin to afferent lymph is vital for the initiation and maintenance of dermal immune responses. How such an exit is achieved and how cells transmigrate the distinct endothelium of lymphatic vessels are unknown. We show that inflammatory cytokines trigger activation of dermal lymphatic endothelial cells (LECs), leading to expression of the key leukocyte adhesion receptors intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin, as well as a discrete panel of chemokines and other potential regulators of leukocyte transmigration VSports手机版. Furthermore, we show that both ICAM-1 and VCAM-1 are induced in the dermal lymphatic vessels of mice exposed to skin contact hypersensitivity where they mediate lymph node trafficking of dendritic cells (DCs) via afferent lymphatics. Lastly, we show that tumor necrosis factor alpha stimulates both DC adhesion and transmigration of dermal LEC monolayers in vitro and that the process is efficiently inhibited by ICAM-1 and VCAM-1 adhesion-blocking monoclonal antibodies. These results reveal a CAM-mediated mechanism for recruiting leukocytes to the lymph nodes in inflammation and highlight the process of lymphatic transmigration as a potential new target for antiinflammatory therapy. .
Figures
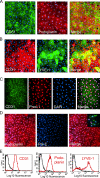
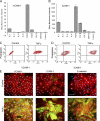
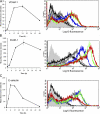
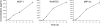
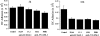
References
-
- Mellman, I., and R.M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell. 106:255–258. - V体育官网 - PubMed
-
- Muller, W.A. 2003. Leukocyte-endothelial cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 24:327–334. - PubMed
-
- Randolph, G.J., V. Angeli, and M.A. Swartz. 2005. Dendritic cell trafficking to lymph nodes though lymphatic vessels. Nat. Rev. Immunol. 5:617–628. - "V体育平台登录" PubMed
-
- Sallusto, F., P. Schaerli, P. Loetscher, C. Schaniel, D. Lenig, C.R. Mackay, S. Qin, and A. Lanzavecchia. 1998. Rapid and co-ordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 28:2760–2769. - PubMed
-
- Saeki, H., A.M. Moore, M.J. Brown, and S.T. Hwang. 1999. Secondary lymphoid tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J. Immunol. 162:2472–2475. - PubMed
Publication types
- "V体育2025版" Actions
MeSH terms
- "VSports手机版" Actions
- VSports注册入口 - Actions
- V体育ios版 - Actions
- V体育官网入口 - Actions
- Actions (V体育安卓版)
- "V体育ios版" Actions
- Actions (VSports手机版)
- "V体育2025版" Actions
- V体育ios版 - Actions
- Actions (VSports最新版本)
- "V体育安卓版" Actions
Substances (V体育2025版)
- VSports在线直播 - Actions
- "VSports最新版本" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

