Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression
- PMID: 17114585
- PMCID: PMC1635149
- DOI: 10.1101/gad.1475506 (VSports最新版本)
Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression
V体育官网 - Abstract
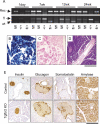
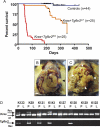
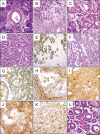
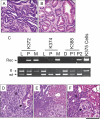
Pancreatic ductal adenocarcinoma (PDAC) is an almost uniformly lethal disease in humans. Transforming growth factor-beta (TGF-beta) signaling plays an important role in PDAC progression, as indicated by the fact that Smad4, which encodes a central signal mediator downstream from TGF-beta, is deleted or mutated in 55% and the type II TGF-beta receptor (Tgfbr2) gene is altered in a smaller subset of human PDAC. Pancreas-specific Tgfbr2 knockout mice have been generated, alone or in the context of active Kras (Kras(G12D)) expression, using the Cre-loxP system driven by the endogenous Ptf1a (pancreatic transcription factor-1a) locus VSports手机版. Pancreas-selective Tgfbr2 knockout alone gave no discernable phenotype in 1. 5 yr. Pancreas-specific Kras(G12D) activation alone essentially generated only intraepithelial neoplasia within 1 yr. In contrast, the Tgfbr2 knockout combined with Kras(G12D) expression developed well-differentiated PDAC with 100% penetrance and a median survival of 59 d. Heterozygous deletion of Tgfbr2 with Kras(G12D) expression also developed PDAC, which indicated a haploinsufficiency of TGF-beta signaling in this genetic context. The clinical and histopathological manifestations of the combined Kras(G12D) expression and Tgfbr2 knockout mice recapitulated human PDAC. The data show that blockade of TGF-beta signaling and activated Ras signaling cooperate to promote PDAC progression. .
Figures


VSports最新版本 - References
-
- Aikawa, T., Gunn, J., Spong, S.M., Klaus, S.J., Korc, M. Connective tissue growth factor-specific antibody attenuates tumor growth, metastasis, and angiogenesis in an orthotopic mouse model of pancreatic cancer. Mol. Cancer Ther. 2006;5:1108–1116. - PubMed
-
- Bardeesy, N., Aguirre, A.J., Chu, G.C., Cheng, K.H., Lopez, L.V., Hezel, A.F., Feng, B., Brennan, C., Weissleder, R., Mahmood, U., et al. Both p16(Ink4a) and the p19(Arf)–p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc. Natl. Acad. Sci. 2006;103:5947–5952. - PMC - PubMed
-
- Bierie, B., Moses, H.L. TGF-β and cancer. Cytokine Growth Factor Rev. 2006;17:29–40. - PubMed
-
- Bottinger, E.P., Jakubczak, J.L., Roberts, I.S., Mumy, M., Hemmati, P., Bagnall, K., Merlino, G., Wakefield, L.M. Expression of a dominant-negative mutant TGF-β type II receptor in transgenic mice reveals essential roles for TGF-β in regulation of growth and differentiation in the exocrine pancreas. EMBO J. 1997;16:2621–2633. - PMC - PubMed
V体育官网入口 - Publication types
MeSH terms
- VSports最新版本 - Actions
- "V体育官网" Actions
- Actions (V体育官网入口)
- Actions (VSports app下载)
- "VSports最新版本" Actions
- "V体育平台登录" Actions
- Actions (V体育安卓版)
- "VSports注册入口" Actions
- VSports - Actions
- "VSports在线直播" Actions
- "V体育ios版" Actions
- "VSports" Actions
- "VSports最新版本" Actions
- "V体育ios版" Actions
- "V体育官网" Actions
- Actions (VSports注册入口)
- Actions (V体育安卓版)
Substances
- "V体育官网" Actions
- Actions (V体育2025版)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
