Dual role of SnoN in mammalian tumorigenesis
- PMID: 17074815
- PMCID: "VSports最新版本" PMC1800653
- DOI: 10.1128/MCB.01394-06
"V体育安卓版" Dual role of SnoN in mammalian tumorigenesis
Abstract (VSports手机版)
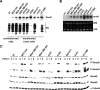
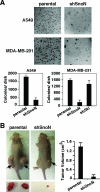
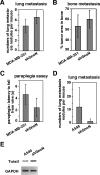
SnoN is an important negative regulator of transforming growth factor beta signaling through its ability to interact with and repress the activity of Smad proteins. It was originally identified as an oncoprotein based on its ability to induce anchorage-independent growth in chicken embryo fibroblasts. However, the roles of SnoN in mammalian epithelial carcinogenesis have not been well defined. Here we show for the first time that SnoN plays an important but complex role in human cancer. SnoN expression is highly elevated in many human cancer cell lines, and this high level of SnoN promotes mitogenic transformation of breast and lung cancer cell lines in vitro and tumor growth in vivo, consistent with its proposed pro-oncogenic role VSports手机版. However, this high level of SnoN expression also inhibits epithelial-to-mesenchymal transdifferentiation. Breast and lung cancer cells expressing the shRNA for SnoN exhibited an increase in cell motility, actin stress fiber formation, metalloprotease activity, and extracellular matrix production as well as a reduction in adherens junction proteins. Supporting this observation, in an in vivo breast cancer metastasis model, reducing SnoN expression was found to moderately enhance metastasis of human breast cancer cells to bone and lung. Thus, SnoN plays both pro-tumorigenic and antitumorigenic roles at different stages of mammalian malignant progression. The growth-promoting activity of SnoN appears to require its ability to bind to and repress the Smad proteins, while the antitumorigenic activity can be mediated by both Smad-dependent and Smad-independent pathways and requires the activity of small GTPase RhoA. Our study has established the importance of SnoN in mammalian epithelial carcinogenesis and revealed a novel aspect of SnoN function in malignant progression. .
Figures
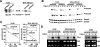




References
-
- Agarwal, C., J. R. Hembree, E. A. Rorke, and R. L. Eckert. 1994. Transforming growth factor beta 1 regulation of metalloproteinase production in cultured human cervical epithelial cells. Cancer Res 54:943-949. - PubMed
-
- Akhurst, R. J., and R. Derynck. 2001. TGF-beta signaling in cancer—a double-edged sword. Trends Cell Biol. 11:S44-S51. - PubMed
-
- Akiyoshi, S., H. Inoue, J. Hanai, K. Kusanagi, N. Nemoto, K. Miyazono, and M. Kawabata. 1999. c-Ski acts as a transcriptional co-repressor in transforming growth factor-beta signaling through interaction with smads. J. Biol. Chem. 274:35269-35277. - PubMed
-
- Attisano, L., and J. L. Wrana. 2002. Signal transduction by the TGF-beta superfamily. Science 296:1646-1647. - PubMed
-
- Azuma, H., S. Ehata, H. Miyazaki, T. Watabe, O. Maruyama, T. Imamura, T. Sakamoto, S. Kiyama, Y. Kiyama, T. Ubai, T. Inamoto, S. Takahara, Y. Itoh, Y. Otsuki, Y. Katsuoka, K. Miyazono, and S. Horie. 2005. Effect of Smad7 expression on metastasis of mouse mammary carcinoma JygMC(A) cells. J. Natl. Cancer Inst 97:1734-1746. - PubMed (V体育ios版)
Publication types
- Actions (VSports在线直播)
MeSH terms
- "VSports最新版本" Actions
- V体育官网 - Actions
- "V体育平台登录" Actions
- Actions (VSports在线直播)
- Actions (V体育平台登录)
- "V体育安卓版" Actions
- "V体育平台登录" Actions
- "VSports最新版本" Actions
Substances
- Actions (V体育ios版)
Grants and funding
LinkOut - more resources
Full Text Sources
"VSports手机版" Other Literature Sources
V体育安卓版 - Medical
