"V体育ios版" The bacterial species definition in the genomic era
- PMID: 17062412
- PMCID: PMC1764935
- DOI: 10.1098/rstb.2006.1920
The bacterial species definition in the genomic era
Abstract (VSports在线直播)
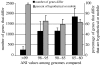
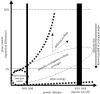
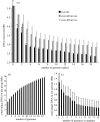
The bacterial species definition, despite its eminent practical significance for identification, diagnosis, quarantine and diversity surveys, remains a very difficult issue to advance. Genomics now offers novel insights into intra-species diversity and the potential for emergence of a more soundly based system. Although we share the excitement, we argue that it is premature for a universal change to the definition because current knowledge is based on too few phylogenetic groups and too few samples of natural populations. Our analysis of five important bacterial groups suggests, however, that more stringent standards for species may be justifiable when a solid understanding of gene content and ecological distinctiveness becomes available VSports手机版. Our analysis also reveals what is actually encompassed in a species according to the current standards, in terms of whole-genome sequence and gene-content diversity, and shows that this does not correspond to coherent clusters for the environmental Burkholderia and Shewanella genera examined. In contrast, the obligatory pathogens, which have a very restricted ecological niche, do exhibit clusters. Therefore, the idea of biologically meaningful clusters of diversity that applies to most eukaryotes may not be universally applicable in the microbial world, or if such clusters exist, they may be found at different levels of distinction. .
"VSports注册入口" Figures


References
-
- Alland D, et al. Modeling bacterial evolution with comparative-genome-based marker systems: application to Mycobacterium tuberculosis evolution and pathogenesis. J. Bacteriol. 2003;185:3392–3399. doi:10.1128/JB.185.11.3392-3399.2003 - DOI - PMC - PubMed
-
- Altschul S.F, Madden T.L, Schaffer A.A, Zhang J, Zhang Z, Miller W, Lipman D.J. Gapped Blast and PSI-Blast: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi:10.1093/nar/25.17.3389 - DOI - PMC - PubMed
-
- Baldwin A, et al. Multilocus sequence typing scheme that provides both species and strain differentiation for the Burkholderia cepacia complex. J. Clin. Microbiol. 2005;43:4665–4673. doi:10.1128/JCM.43.9.4665-4673.2005 - DOI - PMC - PubMed
-
- Boyd E.F, Brussow H. Common themes among bacteriophage-encoded virulence factors and diversity among the bacteriophages involved. Trends Microbiol. 2002;10:521–529. doi:10.1016/S0966-842X(02)02459-9 - DOI - PubMed
-
- Brenner D, Staley J, Krieg N. Classification of prokaryotic organisms and the concept of Bacterial speciation. Springer; New York, NY: 2000. Bergey's manual of systematic bacteriology.
Publication types
- VSports注册入口 - Actions
- V体育ios版 - Actions
MeSH terms
- Actions (V体育ios版)
- "VSports app下载" Actions
- Actions (VSports手机版)
- "VSports在线直播" Actions
VSports注册入口 - LinkOut - more resources
Full Text Sources
VSports手机版 - Other Literature Sources
Molecular Biology Databases

