Impaired regulation of NF-kappaB and increased susceptibility to colitis-associated tumorigenesis in CYLD-deficient mice
- PMID: 17053834
- PMCID: PMC1616194
- DOI: 10.1172/JCI28746
Impaired regulation of NF-kappaB and increased susceptibility to colitis-associated tumorigenesis in CYLD-deficient mice
Abstract
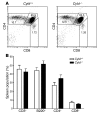
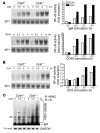
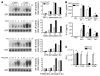
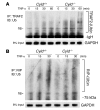
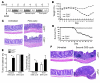
Cylindromatosis (CYLD) is a deubiquitinating enzyme that is altered in patients with familial cylindromatosis, a condition characterized by numerous benign adnexal tumors. However, the regulatory function of CYLD remains unsettled. Here we show that the development of B cells, T cells, and myeloid cells was unaffected in CYLD-deficient mice, but that the activation of these cells with mediators of innate and adaptive immunity resulted in enhanced NF-kappaB and JNK activity associated with increased TNF receptor-associated factor 2 (TRAF2) and NF-kappaB essential modulator (NEMO) ubiquitination. CYLD-deficient mice were more susceptible to induced colonic inflammation and showed a dramatic increase in the incidence of tumors compared with controls in a colitis-associated cancer model. These results suggest that CYLD limits inflammation and tumorigenesis by regulating ubiquitination in vivo. VSports手机版.
"VSports注册入口" Figures


V体育官网 - References
-
- Kovalenko A., et al. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature. 2003;424:801–805. - PubMed
-
- Trompouki E., et al. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. 2003;424:793–796. - PubMed
-
- Brummelkamp T.R., Nijman S.M., Dirac A.M., Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424:797–801. - PubMed
-
- Makarova K.S., Aravind L., Koonin E.V. A novel superfamily of predicted cysteine proteases from eukaryotes, viruses and Chlamydia pneumoniae. Trends Biochem. Sci. 2000;25:50–52. - VSports app下载 - PubMed
-
- Brooke H. Epithelioma adenoides cysticum. Br. J. Dermatol. 1892;4:269–287.
MeSH terms
- Actions (VSports)
- "VSports在线直播" Actions
- "VSports在线直播" Actions
- VSports注册入口 - Actions
- "VSports最新版本" Actions
- VSports最新版本 - Actions
- Actions (VSports最新版本)
- Actions (V体育官网入口)
- V体育安卓版 - Actions
- Actions (VSports最新版本)
- Actions (VSports在线直播)
- "VSports手机版" Actions
- VSports最新版本 - Actions
- "VSports" Actions
- Actions (VSports手机版)
Substances (VSports在线直播)
- VSports - Actions
- Actions (V体育2025版)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
VSports注册入口 - Research Materials
Miscellaneous

