Mll partial tandem duplication induces aberrant Hox expression in vivo via specific epigenetic alterations
- PMID: 16981007
- PMCID: PMC1564428
- DOI: 10.1172/JCI25546
VSports注册入口 - Mll partial tandem duplication induces aberrant Hox expression in vivo via specific epigenetic alterations
Abstract
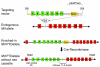
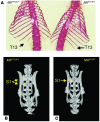
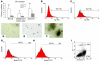
We previously identified a rearrangement of mixed-lineage leukemia (MLL) gene (also known as ALL-1, HRX, and HTRX1), consisting of an in-frame partial tandem duplication (PTD) of exons 5 through 11 in the absence of a partner gene, occurring in approximately 4%-7% of patients with acute myeloid leukemia (AML) and normal cytogenetics, and associated with a poor prognosis. The mechanism by which the MLL PTD contributes to aberrant hematopoiesis and/or leukemia is unknown. To examine this, we generated a mouse knockin model in which exons 5 through 11 of the murine Mll gene were targeted to intron 4 of the endogenous Mll locus. Mll(PTD/WT) mice exhibit an alteration in the boundaries of normal homeobox (Hox) gene expression during embryogenesis, resulting in axial skeletal defects and increased numbers of hematopoietic progenitor cells. Mll(PTD/WT) mice overexpress Hoxa7, Hoxa9, and Hoxa10 in spleen, BM, and blood. An increase in histone H3/H4 acetylation and histone H3 lysine 4 (Lys4) methylation within the Hoxa7 and Hoxa9 promoters provides an epigenetic mechanism by which this overexpression occurs in vivo and an etiologic role for MLL PTD gain of function in the genesis of AML. VSports手机版.
VSports在线直播 - Figures

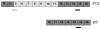

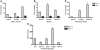

References
-
- Yu B.D., Hess J.L., Horning S.E., Brown G.A., Korsmeyer S.J. Altered Hox expression and segmental identity in Mll-mutant mice. Nature. 1995;378:505–508. - PubMed
-
- Caligiuri M.A., et al. Molecular rearrangement of the ALL-1 gene in acute myeloid leukemia without cytogenetic evidence of 11q23 chromosomal translocations. Cancer Res. 1994;54:370–373. - "VSports手机版" PubMed
-
- Caligiuri M., et al. Rearrangement of ALL1 (MLL) in acute myeloid leukemia with normal cytogenetics. . Cancer Res. 1998;58:55–59. - PubMed
-
- Dohner K., et al. Prognostic significance of partial tandem duplications of the MLL gene in adult patients 16 to 60 years old with acute myeloid leukemia and normal cytogenetics: a study of the Acute Myeloid Leukemia Study Group Ulm. J. Clin. Oncol. 2002;20:3254–3261. - "V体育安卓版" PubMed
Publication types
"V体育安卓版" MeSH terms
- VSports在线直播 - Actions
- Actions (V体育官网入口)
- "VSports注册入口" Actions
- "V体育安卓版" Actions
- "VSports最新版本" Actions
- Actions (V体育2025版)
- "V体育ios版" Actions
- VSports手机版 - Actions
- VSports最新版本 - Actions
- VSports app下载 - Actions
- "V体育安卓版" Actions
Substances
- V体育官网 - Actions
- "V体育官网" Actions
- "V体育2025版" Actions
- Actions (V体育安卓版)
- "V体育官网入口" Actions
Grants and funding
LinkOut - more resources
Full Text Sources (VSports手机版)
Other Literature Sources (VSports注册入口)
Molecular Biology Databases (V体育2025版)
Research Materials

