Tumor necrosis factor alpha- and inducible nitric oxide synthase-producing dendritic cells are rapidly recruited to the bladder in urinary tract infection but are dispensable for bacterial clearance
- PMID: 16966414
- PMCID: PMC1695502
- DOI: V体育官网入口 - 10.1128/IAI.00881-06
Tumor necrosis factor alpha- and inducible nitric oxide synthase-producing dendritic cells are rapidly recruited to the bladder in urinary tract infection but are dispensable for bacterial clearance
"VSports最新版本" Abstract
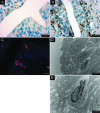
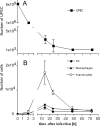
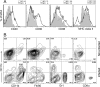
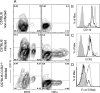
The role of dendritic cells (DC) in urinary tract infections (UTI) is unknown. These cells contribute directly to the innate defense against various viral and bacterial infections. Here, we studied their role in UTI using an experimental model induced by transurethral instillation of the uropathogenic Escherichia coli (UPEC) strain 536 into C57BL/6 mice. While few DC were found in the uninfected bladder, many had been recruited after 24 h, mostly to the submucosa and uroepithelium. They expressed markers of activation and maturation and exhibited the CD11b+ F4/80+ CD8- Gr-1- myeloid subtype. Also, tumor necrosis factor alpha (TNF-alpha)- and inducible nitric oxide synthase (iNOS)-producing CD11bINT DC (Tip-DC) were detected, which recently were proposed to be critical in the defense against bacterial infections. However, Tip-DC-deficient CCR2-/- mice did not show reduced clearance of UPEC from the infected bladder VSports手机版. Moreover, clearance was also unimpaired in CD11c-DTR mice depleted of all DC by injection of diphtheria toxin. This may be explained by the abundance of granulocytes and of iNOS- and TNF-alpha-producing non-DC that were able to replace Tip-DC functionality. These findings demonstrate that some of the abundant DC recruited in UTI contributed innate immune effector functions, which were, however, dispensable in the microenvironment of the bladder. .
Figures


References
-
- Bouchelouche, K., L. Andresen, S. Alvarez, J. Nordling, O. H. Nielsen, and P. Bouchelouche. 2006. Interleukin-4 and 13 induce the expression and release of monocyte chemoattractant protein 1, interleukin-6 and stem cell factor from human detrusor smooth muscle cells: synergy with interleukin-1beta and tumor necrosis factor-alpha. J. Urol. 175:760-765. - PubMed
-
- Brzuszkiewicz, E., H. Brüggemann, H. Liesegang, M. Emmerth, T. Oelschlaeger, G. Nagy, K. Albermann, C. Wagner, C. Buchrieser, L. Emödy, G. Gottschalk, J. Hacker, and U. Dobrindt. 2006. How to become a uropathogen: comparative genomic analysis of extraintestinal pathogenic Escherichia coli strains. Proc. Natl. Acad. Sci. USA 103:12879-12884. - PMC - PubMed
-
- Chan, E. D., J. Chan, and N. W. Schluger. 2001. What is the role of nitric oxide in murine and human host defense against tuberculosis? Current knowledge. Am. J. Respir. Cell Mol. Biol. 25:606-612. - PubMed
-
- Chen, M. C., C. S. Mudge, and D. Klumpp. 2006. Urothelial lesion formation is mediated by TNFR1 during neurogenic cystitis. Am. J. Physiol. Renal Physiol. 291:F741-F749. - V体育ios版 - PubMed
Publication types
MeSH terms
- Actions (VSports手机版)
- "VSports手机版" Actions
- "VSports" Actions
- "VSports在线直播" Actions
- Actions (VSports)
- Actions (V体育ios版)
- Actions (VSports最新版本)
- "VSports注册入口" Actions
- "V体育平台登录" Actions
- "VSports app下载" Actions
- Actions (VSports在线直播)
Substances
- VSports在线直播 - Actions
LinkOut - more resources (V体育2025版)
Full Text Sources
"V体育官网" Medical
Molecular Biology Databases
Research Materials (V体育平台登录)

