Opposing roles of mitogenic and stress signaling pathways in the induction of cancer dormancy
- PMID: 16929185
- PMCID: PMC2517052
- DOI: 10.4161/cc.5.16.3109
Opposing roles of mitogenic and stress signaling pathways in the induction of cancer dormancy
Abstract
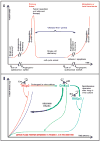
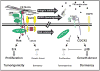
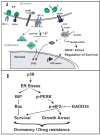
Cancer dormancy is a poorly understood stage of cancer progression. However, the ability to control this step of the disease offers novel therapeutic opportunities. Here we summarize recent findings that implicate the extracellular matrix and adhesion receptor signaling in the escape or induction of tumor dormancy. We further review evidence suggesting that imbalances in the activity ratio of ERK to p38 signaling may determine the fate (i. e. , tumorigenicity vs. dormancy) of different carcinoma cells. Special attention is placed on the mechanisms that p38 signaling regulates during the induction of dormancy and how modulation of these pathways may offer a therapeutic opportunity VSports手机版. We also review evidence for a novel drug-resistance mechanism in dormant tumor cells that when blocked may enable killing of dormant tumor cells. Finally, we explore the notion that dormancy of tumor cells may be the result of a selective adaptive response that allows disseminated tumor cells to pause their growth and cope with stress signaling imposed by dissemination and/or treatment until growth can be restored. .
Figures

"VSports注册入口" References
-
- Meng S, Tripathy D, Frenkel EP, Shete S, Naftalis EZ, Huth JF, Beitsch PD, Leitch M, Hoover S, Euhus D, Haley B, Morrison L, Fleming TP, Herlyn D, Terstappen LW, Fehm T, Tucker TF, Lane N, Wang J, Uhr JW. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004;10:8152–62. - "VSports注册入口" PubMed
-
- Chambers AF, Naumov GN, Varghese HJ, Nadkarni KV, MacDonald IC, Groom AC. Critical steps in hematogenous metastasis: An overview. Surg Oncol Clin N Am. 2001;10:243–55. vii. - PubMed
-
- Fidler IJ. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. - PubMed (VSports app下载)
-
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. - PubMed (VSports注册入口)
-
- Demicheli R, Retsky MW, Swartzendruber DE, Bonadonna G. Proposal for a new model of breast cancer metastatic development. Ann Oncol. 1997;8:1075–80. - PubMed
Publication types
- VSports在线直播 - Actions
MeSH terms
- V体育官网 - Actions
- Actions (V体育ios版)
- "V体育官网" Actions
- VSports - Actions
- VSports最新版本 - Actions
- Actions (V体育平台登录)
- Actions (VSports注册入口)
- "V体育官网" Actions
- VSports注册入口 - Actions
"V体育安卓版" Substances
- "VSports" Actions
- V体育2025版 - Actions
- "VSports" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
