Role of hoogsteen edge hydrogen bonding at template purines in nucleotide incorporation by human DNA polymerase iota
- PMID: 16914729
- PMCID: PMC1592827
- DOI: 10.1128/MCB.00851-06
"V体育官网入口" Role of hoogsteen edge hydrogen bonding at template purines in nucleotide incorporation by human DNA polymerase iota
Abstract
Human DNA polymerase iota (Pol iota) differs from other DNA polymerases in that it exhibits a marked template specificity, being more efficient and accurate opposite template purines than opposite pyrimidines. The crystal structures of Pol iota with template A and incoming dTTP and with template G and incoming dCTP have revealed that in the Pol iota active site, the templating purine adopts a syn conformation and forms a Hoogsteen base pair with the incoming pyrimidine which remains in the anti conformation. By using 2-aminopurine and purine as the templating residues, which retain the normal N7 position but lack the N(6) of an A or the O(6) of a G, here we provide evidence that whereas hydrogen bonding at N(6) is dispensable for the proficient incorporation of a T opposite template A, hydrogen bonding at O(6) is a prerequisite for C incorporation opposite template G. To further analyze the contributions of O(6) and N7 hydrogen bonding to DNA synthesis by Pol iota, we have examined its proficiency for replicating through the (6)O-methyl guanine and 8-oxoguanine lesions, which affect the O(6) and N7 positions of template G, respectively. We conclude from these studies that for proficient T incorporation opposite template A, only the N7 hydrogen bonding is required, but for proficient C incorporation opposite template G, hydrogen bonding at both the N7 and O(6) is an imperative. The dispensability of N(6) hydrogen bonding for proficient T incorporation opposite template A has important biological implications, as that would endow Pol iota with the ability to replicate through lesions which impair the Watson-Crick hydrogen bonding potential at both the N1 and N(6) positions of templating A VSports手机版. .
VSports app下载 - Figures
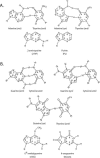
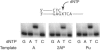

References
-
- Cho, B. P., F. F. Kadlubar, S. J. Culp, and F. E. Evans. 1990. 15N nuclear magnetic resonance studies in the tautomerism of 8-hydroxy-2′-deoxyguanosine, 8-hydroxyguanosine, and other C8-substituted guanine nucleosides. Chem. Res. Toxicol. 3:445-452. - VSports注册入口 - PubMed
-
- Chung, F.-L., L. Zhang, J. E. Ocando, and R. G. Nath. 1999. Role of 1,N2-propanodeoxygunosine adducts as endogenous DNA lesions in rodents and humans. IARC Sci. Publ. 150:45-53. - VSports在线直播 - PubMed
Publication types
V体育官网 - MeSH terms
- "V体育安卓版" Actions
- VSports - Actions
- "V体育2025版" Actions
- Actions (V体育平台登录)
- "V体育官网" Actions
- Actions (V体育官网)
- Actions (V体育官网)
- Actions (VSports)
- Actions (V体育官网入口)
- Actions (V体育平台登录)
"V体育官网" Substances
- "V体育官网入口" Actions
- "VSports注册入口" Actions
- Actions (V体育平台登录)
V体育安卓版 - Grants and funding
LinkOut - more resources
"VSports手机版" Full Text Sources
Miscellaneous
