Cellular senescence in naevi and immortalisation in melanoma: a role for p16? (VSports)
- PMID: 16880792
- PMCID: "V体育ios版" PMC2360676
- DOI: "V体育官网入口" 10.1038/sj.bjc.6603283
Cellular senescence in naevi and immortalisation in melanoma: a role for p16?
Abstract
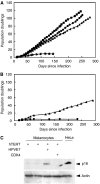
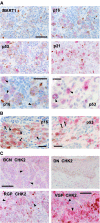
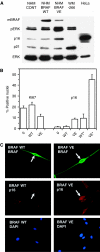
Cellular senescence, the irreversible proliferative arrest seen in somatic cells after a limited number of divisions, is considered a crucial barrier to cancer, but direct evidence for this in vivo was lacking until recently. The best-known form of human cell senescence is attributed to telomere shortening and a DNA-damage response through p53 and p21. There is also a more rapid form of senescence, dependent on the p16-retinoblastoma pathway. p16 (CDKN2A) is a known melanoma susceptibility gene. Here, we use retrovirally mediated gene transfer to confirm that the normal form of senescence in cultured human melanocytes involves p16, since disruption of the p16/retinoblastoma pathway is required as well as telomerase activation for immortalisation. Expression (immunostaining) patterns of senescence mediators and markers in melanocytic lesions provide strong evidence that cell senescence occurs in benign melanocytic naevi (moles) in vivo and does not involve p53 or p21 upregulation, although p16 is widely expressed. In comparison, dysplastic naevi and early (radial growth-phase, RGP) melanomas show less p16 and some p53 and p21 immunostaining. All RGP melanomas expressed p21, suggesting areas of p53-mediated senescence, while most areas of advanced (vertical growth-phase) melanomas lacked both p16 and p21, implying escape from both forms of senescence (immortalisation) VSports手机版. Moreover, nuclear p16 but not p21 expression can be induced in human melanocytes by oncogenic BRAF, as found in around 80% of naevi. We conclude that cell senescence can form a barrier to melanoma development. This also provides a potential explanation of why p16 is a melanoma suppressor gene. .
Figures


"V体育官网入口" References
-
- Bandyopadhyay D, Timchenko N, Suwa T, Hornsby PJ, Campisi J, Medrano EE (2001) The human melanocyte: a model system to study the complexity of cellular aging and transformation in non-fibroblastic cells. Exp Gerontol 36: 1265–1275 - PubMed
-
- Bartkova J, Lukas J, Guldberg P, Alsner J, Kirkin AF, Zeuthen J, Bartek J (1996) The p16-cyclin D/Cdk4-pRb pathway as a functional unit frequently altered in melanoma pathogenesis. Cancer Res 56: 5475–5483 - PubMed
-
- Bastian BC (2003) The longer your telomeres, the larger your nevus? Am J Dermatopathol 25: 83–84 - V体育官网入口 - PubMed
-
- Bennett DC (2003) Human melanocyte senescence and melanoma susceptibility genes. Oncogene 22: 3063–3069 - PubMed
-
- Bennett DC (2006) Familial melanoma genes, melanocyte immortalization and melanoma initiation. In: Melanocytes to Melanoma: The Progression to Malignancy, Hearing VJ, Leong SPL (eds) New Jersey: Humana Press
"VSports手机版" Publication types
MeSH terms
- Actions (VSports手机版)
- Actions (VSports最新版本)
- "V体育官网入口" Actions
- VSports注册入口 - Actions
- Actions (VSports手机版)
- "V体育官网入口" Actions
Substances (VSports)
- VSports最新版本 - Actions
- "V体育2025版" Actions
- VSports app下载 - Actions
- Actions (V体育平台登录)
Grants and funding (V体育ios版)
LinkOut - more resources
Full Text Sources
"VSports在线直播" Other Literature Sources
Medical
Research Materials (V体育官网入口)
Miscellaneous

