"VSports app下载" Loss of Atrx affects trophoblast development and the pattern of X-inactivation in extraembryonic tissues
- PMID: 16628246
- PMCID: PMC1440874
- DOI: 10.1371/journal.pgen.0020058
V体育安卓版 - Loss of Atrx affects trophoblast development and the pattern of X-inactivation in extraembryonic tissues
"V体育ios版" Abstract
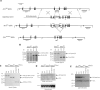
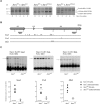
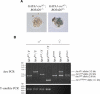
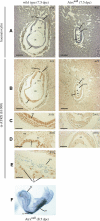
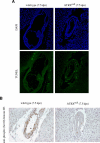
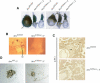
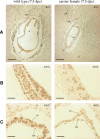
ATRX is an X-encoded member of the SNF2 family of ATPase/helicase proteins thought to regulate gene expression by modifying chromatin at target loci. Mutations in ATRX provided the first example of a human genetic disease associated with defects in such proteins. To better understand the role of ATRX in development and the associated abnormalities in the ATR-X (alpha thalassemia mental retardation, X-linked) syndrome, we conditionally inactivated the homolog in mice, Atrx, at the 8- to 16-cell stage of development. The protein, Atrx, was ubiquitously expressed, and male embryos null for Atrx implanted and gastrulated normally but did not survive beyond 9 VSports手机版. 5 days postcoitus due to a defect in formation of the extraembryonic trophoblast, one of the first terminally differentiated lineages in the developing embryo. Carrier female mice that inherit a maternal null allele should be affected, since the paternal X chromosome is normally inactivated in extraembryonic tissues. Surprisingly, however, some carrier females established a normal placenta and appeared to escape the usual pattern of imprinted X-inactivation in these tissues. Together these findings demonstrate an unexpected, specific, and essential role for Atrx in the development of the murine trophoblast and present an example of escape from imprinted X chromosome inactivation. .
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist V体育安卓版.
VSports最新版本 - Figures

References
-
- Gibbons RJ, Higgs DR. Molecular-clinical spectrum of the ATR-X syndrome. Am J Med Genet. 2000;97:204–212. - PubMed
-
- Gibbons RJ, Suthers GK, Wilkie AO, Buckle VJ, Higgs DR. X-linked alpha-thalassemia/mental retardation (ATR-X) syndrome: Localization to Xq12-q21.31 by X inactivation and linkage analysis. Am J Hum Genet. 1992;51:1136–1149. - V体育ios版 - PMC - PubMed
-
- Gibbons RJ, Picketts DJ, Villard L, Higgs DR. Mutations in a putative global transcriptional regulator cause X-linked mental retardation with alpha-thalassemia (ATR-X syndrome) Cell. 1995;80:837–845. - PubMed
-
- Garrick D, Samara V, McDowell TL, Smith AJ, Dobbie L, et al. A conserved truncated isoform of the ATR-X syndrome protein lacking the SWI/SNF-homology domain. Gene. 2004;326:23–34. - PubMed
-
- Picketts DJ, Tastan AO, Higgs DR, Gibbons RJ. Comparison of the human and murine ATRX gene identifies highly conserved, functionally important domains. Mamm Genome. 1998;9:400–403. - PubMed
Publication types
- V体育官网 - Actions
MeSH terms
- VSports手机版 - Actions
- "VSports" Actions
- VSports注册入口 - Actions
- "V体育2025版" Actions
- "VSports最新版本" Actions
- Actions (VSports)
- Actions (VSports最新版本)
- "V体育官网入口" Actions
Substances
- VSports最新版本 - Actions
- "VSports在线直播" Actions
- "V体育ios版" Actions
Grants and funding
"VSports最新版本" LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

