"V体育官网" GATA-6 regulates semaphorin 3C and is required in cardiac neural crest for cardiovascular morphogenesis
- PMID: 16557299
- PMCID: V体育安卓版 - PMC1409743
- DOI: 10.1172/JCI27363
GATA-6 regulates semaphorin 3C and is required in cardiac neural crest for cardiovascular morphogenesis
Abstract
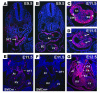
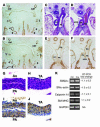
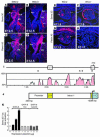
GATA transcription factors play critical roles in restricting cell lineage differentiation during development. Here, we show that conditional inactivation of GATA-6 in VSMCs results in perinatal mortality from a spectrum of cardiovascular defects, including interrupted aortic arch and persistent truncus arteriosus. Inactivation of GATA-6 in neural crest recapitulates these abnormalities, demonstrating a cell-autonomous requirement for GATA-6 in neural crest-derived SMCs. Surprisingly, the observed defects do not result from impaired SMC differentiation but rather are associated with severely attenuated expression of semaphorin 3C, a signaling molecule critical for both neuronal and vascular patterning. Thus, the primary function of GATA-6 during cardiovascular development is to regulate morphogenetic patterning of the cardiac outflow tract and aortic arch. These findings provide new insights into the conserved functions of the GATA-4, -5, and -6 subfamily members and identify GATA-6 and GATA-6-regulated genes as candidates involved in the pathogenesis of congenital heart disease VSports手机版. .
Figures


"VSports" References
-
- Gruber P.J., Epstein J.A. Development gone awry: congenital heart disease. Circ. Res. 2004;94:273–283. - PubMed (VSports最新版本)
-
- Creazzo T.L., Godt R.E., Leatherbury L., Conway S.J., Kirby M.L. Role of cardiac neural crest cells in cardiovascular development. Annu. Rev. Physiol. 1998;60:267–286. - PubMed
-
- Stoller J.Z., Epstein J.A. Cardiac neural crest. Semin. Cell Dev. Biol. . 2005;16:704–715. - PubMed
-
- Feiner L., et al. Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development. 2001;128:3061–3070. - PubMed
-
- Brown C.B., et al. PlexinA2 and semaphorin signaling during cardiac neural crest development. Development. 2001;128:3071–3080. - "V体育官网入口" PubMed
Publication types (VSports)
- VSports - Actions
- VSports注册入口 - Actions
V体育安卓版 - MeSH terms
- "VSports" Actions
- Actions (VSports在线直播)
- Actions (VSports)
- V体育2025版 - Actions
- Actions (V体育2025版)
- VSports注册入口 - Actions
- "V体育官网" Actions
- Actions (VSports手机版)
- "VSports手机版" Actions
- "V体育2025版" Actions
- V体育2025版 - Actions
Substances
- Actions (V体育官网入口)
Grants and funding
LinkOut - more resources
V体育ios版 - Full Text Sources
Molecular Biology Databases

