A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children's Oncology Group
- PMID: 16551859
- PMCID: PMC2587020
- DOI: "VSports注册入口" 10.1158/1078-0432.CCR-05-2000
"V体育官网" A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children's Oncology Group
Abstract (VSports最新版本)
Purpose: Evaluate the clinical safety, toxicity, immune activation/modulation, and maximal tolerated dose of hu14 VSports手机版. 18-IL2 (EMD 273063) in pediatric patients with recurrent/refractory neuroblastoma and other GD2-positive solid tumors. .
Experimental design: Twenty-seven pediatric patients with recurrent/refractory neuroblastoma and one with melanoma were treated with a humanized anti-GD2 monoclonal antibody linked to human interleukin 2 (IL-2). Cohorts of patients received hu14 V体育安卓版. 18-IL2, administered i. v. over 4 hours for three consecutive days, at varying doses. Patients with stable disease, partial, or complete responses were eligible to receive up to three additional courses of therapy. .
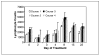
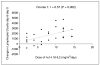
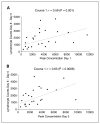
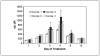
Results: Most of the clinical toxicities were anticipated and similar to those reported with IL-2 and anti-GD2 monoclonal antibody therapy and to those noted in the initial phase I study of hu14. 18-IL2 in adults with metastatic melanoma. The maximal tolerated dose was determined to be 12 mg/m2/d, with agent-related dose-limiting toxicities of hypotension, allergic reaction, blurred vision, neutropenia, thrombocytopenia, and leukopenia. Three patients developed dose-limiting toxicity during course 1; seven patients in courses 2 to 4. Two patients required dopamine for hypotension V体育ios版. There were no treatment-related deaths, and all toxicity was reversible. Treatment with hu14. 18-IL2 led to immune activation/modulation as evidenced by elevated serum levels of soluble IL-2 receptor alpha (sIL2Ralpha) and lymphocytosis. The median half-life of hu14. 18-IL2 was 3. 1 hours. There were no measurable complete or partial responses to hu14. 18-IL2 in this study; however, three patients did show evidence of antitumor activity. .
Conclusion: Hu14. 18-IL2 (EMD 273063) can be administered safely with reversible toxicities in pediatric patients at doses that induce immune activation. A phase II clinical trial of hu14 VSports最新版本. 18-IL2, administered at a dose of 12 mg/m2/d x 3 days repeated every 28 days, will be done in pediatric patients with recurrent/refractory neuroblastoma. .
Figures
References
-
- Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–4. - "VSports手机版" PubMed
-
- Mule JJ, Yang JC, Lafreniere R, Shu SY, Rosenberg SA. Identification of cellular mechanisms operational in vivo during the regression of established pulmonary metastases by the systemic administration of high dose recombinant interleukin-2. J Immunol. 1987;139:285–94. - VSports手机版 - PubMed
-
- Albertini MR, Sondel PM. Tumor immunology and immunotherapy. In: Abeloff MD, Armitage JO, Lichter AS, Niederhuber JE, editors. Clinical oncology. 2. London: Churchill Livingstone; 2000. pp. 214–41.
-
- Colucci F, Caligiuri MA, Di Santo JP. What does it take to make a natural killer? Nat Rev Immunol. 2003;3:413–25. - PubMed
-
- Cheung NK, Sondel PM. Neuroblastoma: immunology and immunotherapy. In: Cohn S, Cheung NK, editors. Neuroblastoma. New York: Springer Press; 2005. pp. 223–42.
Publication types
- V体育ios版 - Actions
- "VSports" Actions
V体育ios版 - MeSH terms
- Actions (VSports注册入口)
- "V体育安卓版" Actions
- Actions (VSports app下载)
- VSports手机版 - Actions
- Actions (VSports app下载)
- Actions (VSports注册入口)
- "V体育ios版" Actions
- "VSports手机版" Actions
- V体育安卓版 - Actions
- "VSports" Actions
- V体育官网 - Actions
- Actions (VSports最新版本)
V体育安卓版 - Substances
- V体育ios版 - Actions
- Actions (VSports app下载)
- "V体育安卓版" Actions
- V体育平台登录 - Actions
Grants and funding
- R01 CA032685/CA/NCI NIH HHS/United States
- M01 RR003186/RR/NCRR NIH HHS/United States
- P01 CA081403/CA/NCI NIH HHS/United States
- V体育平台登录 - P30 CA014520/CA/NCI NIH HHS/United States
- "VSports app下载" CA87025/CA/NCI NIH HHS/United States
- VSports注册入口 - RR03186/RR/NCRR NIH HHS/United States
- 2M01RR01271/RR/NCRR NIH HHS/United States
- "V体育官网入口" M01 RR001271/RR/NCRR NIH HHS/United States
- CA81403/CA/NCI NIH HHS/United States
- VSports手机版 - R01 CA087025/CA/NCI NIH HHS/United States
- CA32685/CA/NCI NIH HHS/United States
- CA14520/CA/NCI NIH HHS/United States
LinkOut - more resources (VSports)
Full Text Sources
Other Literature Sources
Medical

