Streptococcal modulation of cellular invasion via TGF-beta1 signaling
- PMID: 16467160
- PMCID: PMC1413688
- DOI: 10.1073/pnas.0506668103
Streptococcal modulation of cellular invasion via TGF-beta1 signaling
Abstract
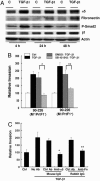
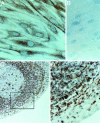
Group A Streptococcus (GAS) and other bacterial pathogens are known to interact with integrins as an initial step in a complex pathway of bacterial ingestion by host cells. Efficient GAS invasion depends on the interaction of bound fibronectin (Fn) with integrins and activation of integrin signaling. TGF-beta1 regulates expression of integrins, Fn, and other extracellular matrix proteins, and positively controls the integrin signaling pathway. Therefore, we postulated that TGF-beta1 levels could influence streptococcal invasion of mammalian cells. Pretreatment of HEp-2 cells with TGF-beta1 increased their capacity to ingest GAS when the bacteria expressed fibronectin-binding proteins (M1 or PrtF1). Western blots revealed significant induction of alpha5 integrin and Fn expression by HEp-2 cells in response to TGF-beta1 VSports手机版. Increased ingestion of streptococci by these cells was blocked by a specific inhibitor of the TGF-beta1 receptor I and antibodies directed against alpha5 integrin and Fn, indicating that increased invasion depends on TGF-beta1 up-regulation of both the alpha5 integrin and Fn. The capacity of TGF-beta1 to up-regulate integrin expression and intracellular invasion by GAS was reproduced in primary human tonsil fibroblasts, which could be a source of TGF-beta1 in chronically infected tonsils. The relationship between TGF-beta1 and GAS invasion was strengthened by the observation that TGF-beta1 production was stimulated in GAS-infected primary human tonsil fibroblasts. These findings suggest a mechanism by which GAS induce a cascade of changes in mammalian tissue leading to elevated expression of the alpha5beta1 receptor, enhanced invasion, and increased opportunity for survival and persistence in their human host. .
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures






References
-
- Kaplan E. L., Johnson D. R. Pediatrics. 2001;108:1180–1186. - PubMed
-
- Gerber M. A., Tanz R. R., Kabat W., Bell G. L., Siddiqui B., Lerer T. J., Lepow M. L., Kaplan E. L., Shulman S. T. Pediatrics. 1999;104:911–917. - PubMed
-
- Osterlund A., Popa R., Nikkila T., Scheynius A., Engstrand L. Laryngoscope. 1997;107:640–647. - PubMed
-
- Park H. S., Francis K. P., Yu J., Cleary P. P. J. Immunol. 2003;171:2532–2537. - PubMed
-
- Bisno A. L., Brito M. O., Collins C. M. Lancet Infect. Dis. 2003;3:191–200. - V体育官网入口 - PubMed
Publication types
- Actions (VSports最新版本)
MeSH terms
- "V体育平台登录" Actions
- "VSports注册入口" Actions
- "V体育平台登录" Actions
- Actions (VSports最新版本)
- V体育官网入口 - Actions
- Actions (V体育官网)
- Actions (VSports最新版本)
Substances (V体育官网)
- "VSports在线直播" Actions
- "VSports在线直播" Actions
- Actions (VSports注册入口)
Grants and funding
LinkOut - more resources
Full Text Sources
V体育安卓版 - Other Literature Sources
V体育安卓版 - Medical
VSports手机版 - Miscellaneous

