Requirement for the SnoN oncoprotein in transforming growth factor beta-induced oncogenic transformation of fibroblast cells
- PMID: 16314499
- PMCID: PMC1316959
- DOI: 10.1128/MCB.25.24.10731-10744.2005
Requirement for the SnoN oncoprotein in transforming growth factor beta-induced oncogenic transformation of fibroblast cells
"V体育平台登录" Abstract
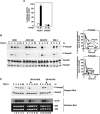
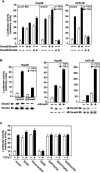
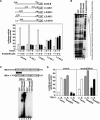
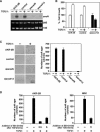
Transforming growth factor beta (TGF-beta) was originally identified by virtue of its ability to induce transformation of the AKR-2B and NRK fibroblasts but was later found to be a potent inhibitor of the growth of epithelial, endothelial, and lymphoid cells. Although the growth-inhibitory pathway of TGF-beta mediated by the Smad proteins is well studied, the signaling pathway leading to the transforming activity of TGF-beta in fibroblasts is not well understood. Here we show that SnoN, a member of the Ski family of oncoproteins, is required for TGF-beta-induced proliferation and transformation of AKR-2B and NRK fibroblasts VSports手机版. TGF-beta induces upregulation of snoN expression in both epithelial cells and fibroblasts through a common Smad-dependent mechanism. However, a strong and prolonged activation of snoN transcription that lasts for 8 to 24 h is detected only in these two fibroblast lines. This prolonged induction is mediated by Smad2 and appears to play an important role in the transformation of both AKR-2B and NRK cells. Reduction of snoN expression by small interfering RNA or shortening of the duration of snoN induction by a pharmacological inhibitor impaired TGF-beta-induced anchorage-independent growth of AKR-2B cells. Interestingly, Smad2 and Smad3 play opposite roles in regulating snoN expression in both fibroblasts and epithelial cells. The Smad2/Smad4 complex activates snoN transcription by direct binding to the TGF-beta-responsive element in the snoN promoter, while the Smad3/Smad4 complex inhibits it through a novel Smad inhibitory site. Mutations of Smad4 that render it defective in heterodimerization with Smad3, which are found in many human cancers, convert the activity of Smad3 on the snoN promoter from inhibitory to stimulatory, resulting in increased snoN expression in cancer cells. Thus, we demonstrate a novel role of SnoN in the transforming activity of TGF-beta in fibroblasts and also uncovered a mechanism for the elevated SnoN expression in some human cancer cells. .
Figures


References
-
- Attisano, L., and J. L. Wrana. 2002. Signal transduction by the TGF-β superfamily. Science 296:1646-1647. - PubMed
-
- Barnard, J. A., R. M. Lyons, and H. L. Moses. 1990. The cell biology of transforming growth factor β. Biochim. Biophys. Acta 1032:79-87. - PubMed
-
- Bonni, S., H. R. Wang, C. G. Causing, P. Kavsak, S. L. Stroschein, K. Luo, and J. L. Wrana. 2001. TGF-β induces assembly of a Smad2-Smurf2 ubiquitin ligase complex that targets SnoN for degradation. Nat. Cell Biol. 3:587-595. - PubMed
-
- Boyd, K. E., and P. J. Farnham. 1999. Coexamination of site-specific transcription factor binding and promoter activity in living cells. Mol. Cell. Biol. 19:8393-8399. - VSports app下载 - PMC - PubMed
-
- Boyer, P. L., C. Colmenares, E. Stavnezer, and S. H. Hughes. 1993. Sequence and biological activity of chicken snoN cDNA clones. Oncogene 8:457-466. - PubMed
Publication types
- Actions (VSports最新版本)
MeSH terms
- V体育平台登录 - Actions
- "V体育平台登录" Actions
- "VSports app下载" Actions
- V体育2025版 - Actions
- V体育平台登录 - Actions
- "VSports手机版" Actions
- "VSports注册入口" Actions
- Actions (V体育2025版)
- "VSports在线直播" Actions
- "V体育官网入口" Actions
- "VSports在线直播" Actions
- V体育官网入口 - Actions
- Actions (VSports在线直播)
Substances
- "VSports手机版" Actions
- "VSports最新版本" Actions
- VSports手机版 - Actions
- Actions (V体育官网入口)
- "V体育安卓版" Actions
Grants and funding
LinkOut - more resources
V体育官网 - Full Text Sources
Other Literature Sources
V体育安卓版 - Miscellaneous
